. Introduction
The first report on the presence of cell-state oscillations in the cambia of trees appeared in the second half of the twentieth century. It was 50 years ago when the longest biological rhythm of cell inclination change, with a period approximating 20 years, was discovered and thoroughly characterized by Hejnowicz (1971, 1973). However, little is known about the physiological or molecular genetic nature of such a rhythm. Cycling cambial cells change their inclination relative to the stem axis in one direction for approximately 10 years and then begin tilting in opposite direction through oriented intrusive growth and anticlinal cell divisions (Hejnowicz, 1971; Hejnowicz & Zagórska-Marek, 1974). These elongated, ever-dividing and growing meristematic cells represent a special category of plant cells equivalent to animal stem cells. The oscillation-phase shift in the population of cycling cells, which occurs along the vertical axis of the cambial cylinder, produces the effect of a moving structural wave, which, in turn, leads to wavy wood formation. The function of these changes is not clear, especially because the resulting longer transport route in wavy patterns becomes energetically expensive. This intriguing question awaits an answer.
Whether cycling cambial cells are driven by an unknown intrinsic oscillator, or whether they are recipients of positional information coming from the external morphogenetic field (morphogenetic wave) traveling over the surface of cambial tissue remains unknown. The first possibility is suggested by the intrinsic cell chirality that has been documented in animal cells and intensely studied in recent years because it plays an important role in the development of the right- and left-handedness of the animal body (Fan et al., 2019; Inaki et al., 2016, 2018; Wan et al., 2011, 2016). Plant cells are yet to be shown to possess similar mechanisms, in the context of the many chiral phenomena observed in plant development (Zagórska-Marek, 2021). The cells are capable of changing their polarity in response to external cues; however, initial reports showed that plant protoplasts developing out of tissue context are equipped with intrinsic mechanisms based on the presence of the cell polarity protein BASL (Chan et al., 2020). The position of the protein is dynamic in an isotropic environment but when fixed, it allows the cells to polarize their growth. Interestingly, once set, the chiral configuration of the plant cell may be maintained for a long time, as shown in the case of apical cell divisions in moss gametophores (Zagórska-Marek et al., 2018).
The second possibility is supported by the fact that morphogenetic waves have different amplitudes, lengths, and velocities, and also, by the fact that they are capable of superposition (Hejnowicz, 1974). Furthermore, such waves were later shown to have the potential to generate various cambial wavy phenotypes, namely, permanently vertical, modulated, or permanently slanted. This diversity results from the mode of a single wave emergence in cambial tissues (Zagórska-Marek, 1995). One of the strongest arguments supporting the idea of a morphogenetic field is the observation that horizontal dislocations in the wavy pattern do not change while traveling along the cambial surface (Zagórska-Marek, 1995).
Cambial waves discovered and characterized by Hejnowicz belong to the category of transverse waves (Walsh, 2022), in which case, cells oscillate between left and right orientation in the plane of the cambial cylinder surface, i.e., perpendicular to the direction of wave propagation. The structural waviness resulting from these developmental changes looks flat when viewed from outside the tree, whereby, in general, it can be classified as a transverse tangential wave.
However, in some trees, especially those with smooth barks, such as beeches, another type of periodic pattern may be noted, which is composed of pronounced, regularly alternating crests and troughs that give the impression that the surface of the tree trunk has rippled (Zagórska-Marek, 1995). Such appearance suggests that spatial changes in cell inclination occur, not in a tangential plane but in a radial plane.
This “start-up” research aimed to determine the general nature of this phenomenon. Does it have taxonomic value as an important species-specific trait? What triggers its emergence? Are there any dynamics in the development of the ripple pattern, and, what remains behind it? Finally, is this radial waviness in any way related to the earlier discovered and now well-known tangential waviness? Although preliminary, the results summarized herein show that the phenomenon is more interesting than originally thought and deserves further investigation.
. Material and Methods
The search for trees with a rippled trunk surface has continued over the past 3 decades in the forests of Europe, Asia, and North America. Superficial observations and photographic documentation were the only possibilities in most cases. The most informative wood samples, suitable for closer morphological and anatomical analyses, were obtained in 2003 from the main stem of a Clethra barbinervis tree growing in Kurama forest near Kyoto, Japan, and in 2006, from the main stem of a 120-year-old beech tree (Fagus sylvatica) fallen in the city of Wrocław, Poland. A rectangular sample (8 cm × 14 cm × 3 cm) was isolated from a living Clethra tree using a shallow tangential cut. The old beech tree provided a large disk, transversely cut, 30 cm high and 53 cm in diameter. The upper and lower sides were labeled. First, it was analyzed and photographed in toto and then split into quadrants. The quadrant with the most distinct ripple pattern was divided by a transverse cut into two smaller disks: the lower, 19 cm high, and the upper, 11 cm high. Both transverse surfaces of the upper disk were smoothened and finely polished to expose the pattern of annual rings. This was the most consistent and well-defined material for analysis of the ripple pattern in a beech tree. Some smaller samples of figured beech wood were collected over the years from timber trees fallen by foresters in the remnants of the old beech forest covering the Trzebnickie Hills north of Wrocław (near the village of Przecławice). In some cases, the apical direction could be detected based on ascending traces of lateral buds or branches overgrown by successive wood deposits, while in others, their position within the tree trunk, and thus their apical–basal polarity, could not be established. However, they were all useful for estimating the overall morphology of the ripple pattern. Representative samples of rippled wood were deposited in the herbarium of the University of Wrocław.
For microscopic analysis, small rectangular wood blocks were excised from Clethra wood along the radius. They were subsequently prepared for sectioning by boiling in water with glycerol for 3 days. The transverse and radial sections were prepared using a sliding microtome (Leica S14 2000), mounted on glass, stained with toluidine blue, and analyzed under an Olympus BX50 epifluorescence microscope equipped with an Olympus DP71 digital camera and Cell^B software. Images of the sections were stored in portable memory carriers. Transverse sections of Clethra and Fagus rippled wood made from areas of wider and narrower rings were double-stained with safranin and Astra Blue and examined for possible differences in cell wall composition.
. Results
Geographic Distribution
Radial waviness was noted and recorded in representative individuals of various tree species growing in different locations on three continents: Europe, Asia, and North America (Table 1, Figure 1). It was especially conspicuous in beeches, most likely because of the smoothness of their bark. The appearance of a ripple pattern in other species was accidental and could not be attributed to any of them as a typical, species-specific trait.
Table 1
Cases of radial waviness recorded in a study.
General Morphology
The ripples visible on the bark were also present on the tangential surface of the wood after the bark was stripped off, indicating that their source was inside the tree, most likely in the cambium. This was best observed in Clethra trees grazed on by deer, but also in beech wood samples devoid of bark. The pattern consisted of rounded crests and troughs, either transverse or inclined relative to the stem axis. The orientation of ripples could be unequivocally determined, also for pieces of wood with an unknown original position within a tree, because it is the same, regardless of the sample’s apical–basal polarity. Ripple orientation, if present, was predominantly to the right (Z). However, at least in one beech wood sample, two patterns were observed: one with right-slanted (Z) and one with left-slanted (S) ripples. These two patterns were circumferentially separated from each other.
Figure 1
Four individual tree trunks, each with a distinct ripple pattern. (A) Fagus sylvatica, Jastrowiec, Poland; (B) Picea abies, Sudety Mts, Poland (photo: Piotr Kiciński); (C) Ailanthus altissima, Brussels, Belgium; (D) Magnolia acuminata, southern Ontario, Canada (photo: Anita Figlar); ripples are right-slanted in (A,B), and left-slanted in (D).

The two investigated Ailanthus trees also had two sets of oppositely inclined ripples that were, however, not separated, as in the beech tree, but superimposed, forming a reticulate pattern on the tree surface (Figure 1C). Notably, an identical structure developed in two trees growing in two very different and distant locations (Table 1). The reticulate pattern among the other investigated species was observed only once in one individual tree of Acer pseudoplatanus. The vertical distance between two consecutive ripples varied from a few centimeters in Clethra and Pyrus to 25 or 30 cm in Picea and Fagus. The greatest distance was noted in Ailanthus (approximately 50 cm). In Fagus, the vertical distance between consecutive ripples varied from 3 to 30 cm, most commonly being approximately 10 cm.
Beech trees provided most examples of ripple patterns. The phenomenon was relatively frequent in straight, vertically growing stems of different sizes; thus, it did not seem to be associated with mechanical forces acting on the tree or with age. There was no clear preference for pattern appearance concerning geographic cardinal direction or tree location. On the surface of a single tree trunk, the number of visible crests and troughs varied longitudinally from a few to so many that they covered the entire length of the stem (Figure 1A,B). The distance between consecutive crests was relatively constant within each pattern. However, it varied among individual cases. Typically, the pattern was circumferentially limited to one side of the tree trunk. The vertical border between the area where the pattern was present and the rest of the circumference of the tree was very sharp (Figure 1A).
Superficial observations did not provide information on whether the pattern was stationary or dynamic, in the sense that each trough in time would be replaced by a crest and vice versa. To resolve this problem, a sufficient number of rippled wood samples had to be collected for closer developmental analysis and microscopic examination.
Radial Pattern Dynamics
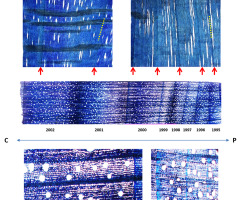
The transverse upper surface of the disk quadrant of the beech wood, in which the ripple pattern was distinct, revealed that it was not stationary (Figure 2). Its dynamics indicated the periodic changes in the width of the annual tree rings along the radius. Approximately 10 years of wider rings in a particular location were followed by nearly 10 years of narrower rings, followed by a period of again wider rings (Figure 2B). These changes occurred at regular intervals. Tracking the same annual ring along the circumference showed that being wide in one location, it became very thin at some distance, and vice versa. This spatial and circumferential periodicity resulted from the fact that the crests and troughs of the ripple pattern were inclined to the longitudinal axis of the tree trunk. The direction of the circumferential shift of specific areas with either wider or narrower annual rings, observed on the transverse surface of the disk, allowed the determination of the direction in which the pattern was moving. In this particular case, they shifted counterclockwise in consecutive years (Figure 2A), implying that Z-oriented crests and troughs of the pattern migrated downward. Another smaller beech wood sample with apical–basal polarity well defined by the presence of a lateral branch showed the opposite situation to the one described above. The areas with either wider or narrower annual rings shifted clockwise; thus, the Z-inclined ripples in the sample moved upward (Figure S1). A similar pattern of regular changes in annual ring width in time and space visible on the transverse cut face of Cryptomeria japonica log (Figure S2) suggested that the observed ripple pattern dynamics are universal, not limited to broadleaved trees.
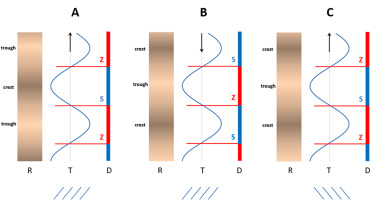
Based on all the above observations, the conclusion is that unequivocally reading the direction of ripple pattern propagation requires data on (i) wood sample apical–basal polarity, (ii) orientation of ripples on the wood tangential face, and (iii) the direction of lateral shift of the areas with either increased or reduced annual ring width on the transverse surface of the wood. The correctness of this interpretation was supported by theoretical analysis, the results of which are shown in Figure 3.
Figure 2
Polished upper surface of a beechwood sample with Z-oriented ripples; (A) sinusoidal outline of the sample edge marks the presence of circumferentially neighboring crests (red dot) and throughs (blue dot). The pattern of the annual rings shows the areas of ring widening and narrowing shifting counterclockwise in time; this means that the ripple pattern migrated downward; (B) magnified view of the ring pattern show sequences of narrower (blue) and wider (red) rings along radial cracks marking the course of wood rays; red and blue dots show how the same annual ring wide in one location becomes narrow in the neighboring area. Scale bars: 10 mm.
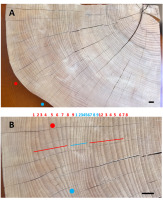
Figure 3
Scheme showing the direction of lateral displacement of the marker (red dot) in the ripple pattern moving downward in the upper panel and upward in the lower one; in both cases the marker shifts to the right because of the opposite orientation of ripples in both patterns: Z in the upper panel and S in the lower one.
It is noteworthy that if the crest and troughs of the ripple pattern were horizontal, as in the Clethra tree shown in Figure 4, the direction of the pattern propagation could not be deduced from the surface of one single transverse section through the stem.
Radial Versus Tangential Waviness
The wood sample collection used in this study comprised 19 (13 + 6) pieces of beech wood and one piece of Clethra wood. In the six beechwood samples, only tangential waviness was observed. Two of them showed a unique phenotype of modulated waviness. Meanwhile, in the other four, the tangential waviness was permanent with notably varying wavelengths, sometimes even within the same piece. All the remaining samples, including Clethra wood, showed Z-oriented ripples accompanied by regular deviations of wood grains from the vertical course in a tangential plane, indicating the superimposition of two waves: the transverse tangential wave (T-wave) and the radial wave (R-wave). The lengths of the waves, as well as their velocity and propagation vectors, were strongly correlated. This was particularly clear on the radial split face of the wood, where the inclination of characteristic linear undulations to the rays showed that the tangential wave migrated at the same speed and in the same direction as the radial wave (Figure 5). Cases of upward and downward migration were recorded in five wood pieces with known apical–basal polarity (Figure 6).
The orientation of the wood grain above and below the summit of each crest was opposite, but their sequence differed depending on the direction of wave propagation. It was S/Z in the case of downward movement, and Z/S for waves propagated acropetally.
Figure 5
Radial (A) and tangential (B) face of a splinter detached from the rippled beech wood showing spatial-temporal dynamics and superposition of R-wave and T-wave; synchronized movement of both waves is acropetal; (A) wood grain, inclined initially towards the pith changes inclination continuously towards the surface of the stem (here on the right side of the sample) or vice versa; this way crests give way to throughs and throughs to crests while moving upward; (B) tangential view of grain deviations from the vertical axis to the left (S) and the right (Z) in the outermost wood layer; they result from the presence of transverse T-wave; vertical sequence of the areas with opposite grain orientation atop of the crest is Z/S; the upper edge of the splinter is apical. Scale bar: 22 mm, the same for (A) and (B).
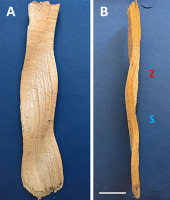
Figure 6
Radial split faces of two beech wood samples with superimposed radial and tangential waviness; in the left sample both periodic patterns moved upward, while, in the right one – in the opposite direction; especially in the right sample superposition of T-wave and R-wave is very clear because of their amplitude – wood grain considered in 3D is inclined not only away and towards the viewer but also away and towards the pith, which in both samples is located on their right side; the apical–basal polarity of both samples is concordant, and their upper transverse surfaces are apical. Scale bar: 70 mm.

The pieces of unknown apical–basal polarity were arbitrarily positioned, assuming an upward movement of the T-wave. In all cases, the wood grain course in the tangential plane was Z-oriented above and S-oriented below the crest. An interesting exception was found in a beech wood sample, in which two separate ripple patterns differed in the orientation of their crests, even though they both migrated in the same direction (Figure 7). This was the only recorded case of the presence of S-oriented ripples in beech. On the summit of the S-oriented crests, the vertical sequence of grain deviations from the longitudinal axis was S/Z, whereas in the second pattern, with Z-oriented crests, it was Z/S, as in all remaining patterns. As mentioned above, a T-wave is not necessarily accompanied by an R-wave. The opposite situation suggests the case of a living Clethra tree with numerous horizontal ripples (Figure 4). There was no evidence of grain deviation from the vertical course of the tree surface.
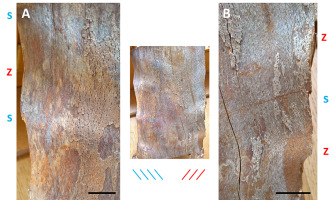
Figure 7
Outermost tangential face of the beechwood sample with two separate ripple patterns (middle photo); the ripples are S-oriented in the left pattern (A) and Z-oriented in the right one (B); both patterns differ in the sequence of opposite grain deviations atop of their crests; it is S/Z for the left pattern and Z/S for the right one. Scale bars: 25 mm.
The tangential waviness in the wood grain course originates in the cambium, where the structural wave in the arrangement of fusiform initials results from the presence of a moving domain pattern (Hejnowicz, 1971, 1974; Hejnowicz & Romberger, 1973). Most probably, the radial wave also arises there, presumably because of the different rates of cell proliferation. The close relationship between the two types of wavy wood patterns posed the following question: How is the production of wider annual rings related to migrating domains – the areas of unidirectional cell-inclination change? Considering the direction of pattern propagation, the sequence of changes in the ring width, and grain orientation in a tangential plane atop the crests, it was possible to determine that, in most cases, the annual rings widened with the onset of the S domain in cambium narrowing when the S domain weakened to be replaced with the Z domain (Figure 8A,B). Only one exception was found in beech wood with S-oriented ripples (Figure 7A, Figure 8C), in which case, the onset of the Z-domain promoted the formation of wider rings.
Figure 8
The relationship between the radial wave (R), the tangential wave (T), and the cambial domain pattern (D) in three variants (A–C) found in this study. All patterns are shown en face; (A) the most common variant, in which the patterns move upward and the grain course changes in a Z/S vertical sequence; (B) the less frequent variant, in which the patterns move downward, opposite to what happens in (A). Both (A) and (B) variants occurred when ripples were Z inclined; (C) a unique variant associated with S-inclined ripples. The borders between consecutive cambial S (blue) and Z (red) domains are at the levels of maximum inclination of wood grain within the T-wave. Arrows show the direction of synchronized movement of all patterns.
The last question relates to the cellular and structural basis of the spatiotemporal changes in the width of annual rings. Investigating rippled wood anatomy is necessary to obtain an answer. This was performed using Clethra wood isolated from a tree with Z-inclined ripples (Figure 9) and a small beechwood sample taken from the main disk (Figure 2).
Microscopic Analysis of Annual Ring Width
Theoretically, there could be two reasons for the regular alternation of sequences of wider and narrower annual rings within the dynamic ripple pattern: hyperplasia and hypertrophy. The analysis of Clethra wood cross sections (Figure 10) revealed that the number of cells in one radial row making up the wide ring was much larger than that in the narrow row. The number spanned between 270 and 12 for the widest and narrowest rings, respectively. This finding shows that cell proliferation in the cambial zone, measured by the frequency of periclinal divisions, was much higher during the formation of wide rings. No signs of cell enlargement were observed in wide rings. Some additional tests were made to check for tension wood characteristics, which if present would indicate the involvement of biomechanical forces in ripple pattern formation. Staining with safranin and Astra Blue showed that thick-walled wood fibers in Fagus and fiber tracheids in Clethra had fully lignified cell walls with no detectable differences between the narrow and wide rings (Figure S3). In addition, the thickness of these walls, as well as the frequency and lumen diameter of the vessels, were the same in both ring types. These results showed no tension wood traits in wider rings.
Figure 10
The anatomy of Clethra rippled wood. The transverse section in the middle of the figure shows a transition from thinner to wider annual rings during the past 8 years; an increase in a ring width during the last 3 years led to the formation of a crest; on the radial sections (upper panel) annual ring borders (broken lines and red arrows) tilt towards the pith (p) when annual rings are wide while they tilt towards the cambium (c) in the area of narrower rings of the through. A magnified view of differentiated xylem cells arranged in the radial rows (bottom panel) exposed their similar size but different numbers in wide and narrow rings. The 2002 annual ring width is 6 mm.
. Discussion
This study showed that the intriguing long-term biological rhythms leading to figured wood formation in tree ontogeny are far more complex than previously thought. The discovery of radial waviness, equally ephemeral as tangential waviness, makes it clear that some dendrochronology studies may have to consider this phenomenon. Its unpredictable appearance in various broad-leaved and coniferous tree species growing at strikingly different locations suggests the existence of an unknown, universal regulatory mechanism. The ability of the cambium to regulate radial growth in a spatio-temporal-dependent manner and to produce wider and narrower annual rings of xylem in approximately 10-year cycles may be of intrinsic nature. Although wider rings are formed in tension wood, the wood anatomy of wide and narrow annual rings associated with radial waviness in Clethra and Fagus was the same. This finding excluded the involvement of external mechanical forces, such as tree bending, in ripple pattern formation. The tension wood hypothesis a priori seemed unlikely because of the spatial periodicity and dynamics of the radial pattern. For these reasons, here, it is proposed to be regulated internally and independently of any external environmental cues, such as climatic changes (Douglass, 1919; Fritts, 1966; Trouet, 2020) or defoliation caused by herbivores or pests, such as spruce budworm (Choristoneura). These factors have always been considered the most important for dendrochronologists developing their barcoding tools for time-scale investigations.
Differences in cell numbers within annual rings associated with the radial wave postulated to be a newly discovered morphogenetic field traveling in the cambium, raise questions regarding the nature of the regulatory mechanism involved. What could precisely control cambial activity in consecutive growing seasons or within the same season in different locations of the cambial surface? The question is difficult to answer. The differences in the number of periclinal divisions might be an effect of positional information in the suggested regulatory field, which induces cambial initials to divide more or less frequently. It is plausible that auxin, the major growth hormone transported in the cambium, may be involved. Endogenous auxin, transported basipetally from flushing buds, initiates cambial activity after winter dormancy (Little & Bonga, 1974). However, even in the absence of endogenous hormone flow from above dormant cambial cells begin dividing periclinally after being locally heated (Oribe & Funada, 2017). They must also be receptive to the presence of auxins (Baba et al., 2011; Little & Bonga, 1974). Can auxin be responsible for the modulated response of cambial cells in terms of their productivity within the morphogenetic field of R-waves? The dividing cells reacting to positional information do not act in a binary mode; rather, their activity changes gradually between opposite states. Is this due to different continuously oscillating auxin concentrations? Is it possible that an elevated or a low auxin concentration is maintained in a particular region of the cambium for approximately 10 years?
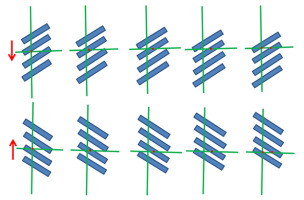
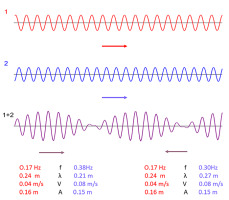
Changes in auxin concentrations in a wavy pattern in the cambium have been reported by Wodzicki (Wodzicki & Wodzicki, 1981; Wodzicki et al., 1979, 1987, 1988; Zajączkowski et al., 1984). These auxin waves were small in length. In a series of ingenious experiments, Wodzicki has shown that wave amplitude may increase significantly when modulated by phytohormones. Theoretically, there is a possibility of the superposition of small waves leading to the formation of periodic patterns (waves) with characteristics that are quite different from those of elementary waves (Figure 11). The patterns may have different lengths of the wave groups and different speeds of their migration, but most importantly the direction of wave group propagation may be opposite to that of the elementary waves. This is worthy of noting in light of the fact that auxin in cambium is transported downward whereas both long-term waves: T-wave and R-wave predominantly move upward.
Figure 11
Simulation of interference between two traveling waves, leading to the emergence of a periodic pattern composed of wave groups. A small change in the length and frequency of only one of the elementary waves alters the direction of pattern propagation. Generated with the use of an interactive program available online (The Physics Classroom, 2022).
This hypothesis of wave interference during auxin flow in the cambium, which bridges the gap between the small length auxin waves and the long-term wave phenomena, is highly speculative, and at present lacks any support from solid experimental data. Otherwise, in the case of tangential waves, Hejnowicz (1974) found that in the cambium of Fraxinus the superposition of two traveling tangential waves differing in length and velocity but with the same period, produced the effect of pulsation in the domain length in a stationary interference pattern. This finding has an interesting consequence. If the period of the elementary waves differed, the interference pattern would start to move. It is already known that tangential waves may have periods shorter than 20 years. The annual rhythm of cell inclination change has been reported in the cambium of the African tree, Daniellia sp. (Détienne, 1979), and the early ontogeny of Cinnamomum camphora (Fujita & Zagorska-Marek, 2005). It is possible that these elementary, small tangential waves interfere leading to the emergence of long-term waves. Based on the above theoretical considerations, it must be remembered that it is still not known whether the concentration of auxin indeed varies between cambial areas where cells proliferate more intensely and those where this activity is much lower.
The regulatory pathways involved in R-wave formation may be complex. In addition to auxin, other phytohormones and many genes are known to regulate the intensity of cambial cell proliferation (Barra-Jiménez & Ragni, 2017; Nieminen et al., 2015; Wang et al., 2021). The most promising finding is that the modulated expression of two MADS-box genes, VCM1 and VCM2, regulates cambium proliferation activity in poplar by tuning the auxin concentration in the tissue (Zheng et al., 2021). Determining how xylem production can increase through internal regulation may have a significant impact on tree biomass production.
Apart from the causes of ripple pattern formation, the most intriguing is its relation to tangential waviness. In particular, the length of its cycle of thinner and wider rings is approximately 20 years, similar to the full cycle of cambial cell inclination change in the plane of the cambial surface. The R-wave, together with the T-wave, belongs to the longest biological rhythms on Earth. The mechanisms underlying these clocks are unknown. It is also difficult to understand why in the majority of investigated cases, one domain sequence in the cambial domain pattern – S following Z in this study – should promote a higher rate of periclinal cell divisions in the cambium, whereas the other sequence – Z following S – would slow them down. The collected material used in this study is likely insufficient to confirm this finding as a rule, especially in light of the single case of beech wood in which the S-inclined ripples showed an opposite relationship between the R-wave and T-wave in terms of a vertical sequence of S- and Z-oriented wood grains atop the crests. Magnolia acuminata, which showed S-oriented ripples in two individual trees (Table 1), would be very useful for checking this behavior, which is unusual in beech trees. Unfortunately, no wood samples were available from these trees for this study.
This research hints at the existence of an entirely new, possibly totally intrinsic mechanism that regulates the width of annual tree-growth rings independently from environmental cues. This is important for those who rely on dendrochronological scales. There are still many unanswered questions connected to the discovery of R-waves, a new, very long biological rhythm. These findings warrant further research.
. Supplementary Material
The following supplementary material is available for this article:
Figure S1. Upper transverse surface of the beechwood sample with Z-oriented ripple pattern moving upward.
Figure S2. Cryptomeria japonica – The bottom surface of the fallen tree log reveals some sequences of wider and thinner annual rings shifting laterally along the circumference.
Figure S3. Microscopic view of the transverse sections through secondary xylem of Clethra and Fagus.




