. Introduction
Somatic embryogenesis (SE) is a plant-specific process of non-sexual reproduction in which differentiated somatic plant cells undergo embryogenic transition and form bipolar structures called somatic embryos. SE can occur naturally in planta in a small number of species, such as Kalanchoe (Crassulaceae). However, somatic embryo formation can be induced in vitro in many plants belonging to various taxonomic groups. Since the first work on SE in carrots in the late 1950s, it has been widely demonstrated that this process can be efficiently triggered by exposing explants to plant growth regulators or stress treatments. Exogenous and endogenous factors that affect SE induction and somatic embryo development have been studied intensively in different species. Numerous protocols that enable the efficient regeneration of dozens of plant species via SE have been established. As a result, SE has become an important biotechnological system that provides efficient in vitro mass propagation of plants for marketing, germplasm conservation, and genetic improvement. In addition to species of commercial value, SE induction methods have been established for the main plant models in genomics, including Arabidopsis thaliana (L.) Heynh., Medicago sativa L., and M. truncatula Gaertn. SE studies in these plants have contributed substantially to a better understanding of the molecular determinants of the somatic-to-embryogenic transition and, in general, the mechanisms of cell totipotency. One of the exciting questions that must be answered is the extent of similarity between somatic and zygotic embryogenesis. Recent findings suggest that despite apparent morphological similarities between somatic and zygotic embryos, embryogenic development initiated by divisions of the plant somatic cell and the fertilized egg (zygote) might differ substantially at the genetic and epigenetic levels.
This review summarizes the achievements of Polish research groups in SE studies and their contributions to the major issues in current plant biology, genomics, and biotechnology. The diverse SE systems developed for several plants (gentians, the tree fern Cyathea delgadii Sternb., and conifers) and plant model species (A. thaliana and Medicago spp.) in Poland are presented, and the applications of these systems are demonstrated.
. Historical Perspective on Somatic Embryogenesis Research in Poland and Its Importance
Crop Plants
The broad application of SE in basic and applied research has stimulated studies on this process in several laboratories worldwide. The first report describing plant propagation via SE in Poland was published in 1982. This research was initiated by Stefan Malepszy at the Department of Plant Genetics, Warsaw Agricultural University, and was concerned with Cucumis sativus L., a vegetable of economic importance (Malepszy et al., 1982). Over the next few years, a cucumber embryogenic suspension culture system was developed (Malepszy & Solarek, 1986), and the phenomenon of natural fluorescence occurring in these cultures was suggested as a marker for the selection of heterokaryocytes obtained by protoplast fusion (Burza et al., 1994). Twenty years of research have resulted in improving the cucumber SE protocol and developing efficient transformation systems. Further research by the team extended to other plant species and resulted in several successes in describing the genetic determinants of responses to biotic and abiotic stimuli.
Three years after presenting the SE method for cucumber, the first paper describing SE in monocotyledons was published by Jan Rybczyński and Janusz Zimny (Rybczyński & Zimny, 1985). The research was initiated at the Polish Academy of Sciences Botanical Garden – Center for Biological Diversity Conservation in Powsin (PAS BG-CBDC). Subsequent studies were published on cereals such as Secale (Rybczyński & Zduńczyk, 1986; Zimny & Lörz, 1986), triticale (Stolarz & Lörz, 1986; Zimny & Rybczyński, 1986), and Hordeum (Rybczyński et al., 1986), which are the most important crops for human and animal nutrition. These pioneering achievements on a global scale became the basis for further highly advanced research on genetic modification and GMOs conducted currently at the Department of Plant Biotechnology and Cytogenetics at the Plant Breeding and Acclimatization Institute (IHAR) – National Research Institute by Janusz Zimny and his team (Zimny & Sowa, in press).
Ornamental plants are the second major group of plants for which SE provides opportunities for large-scale propagation. This process was described for the first time at the University of Agriculture in Krakow by Anna Bach in Freesia (Bach, 1992) and Gentiana pneumonanthe (Bach et al., 1994), as well as at the PAS BG-CBDC by Mikuła and Rybczyński (Mikuła & Rybczyński, 1994) in three other species belonging to the genus Gentiana. Works initiated by Anna Bach and continued by Bożena Pawłowska resulted in progress in propagating bulbous plants and in vitro tools to protect Polish flora (Pawłowska & Ptak, 2022). Another thriving research center was established at the State Research Institute of Horticulture in Skierniewice, where many protocols were developed for propagating various ornamental plant species. Here, a group of researchers consisting of Eleonora Gabryszewska and Małgorzata Podwyszyńska developed protocols (among others, based on SE) that are important for the development of horticulture in Poland. Podwyszyńska et al. (2022) described these findings in a separate study. On the other hand, the development of a suspension culture system for gentians at the Department of Plant Conservation Biology, PAS BG-CBDC, brought new knowledge to experimental plant biology. Embryogenic cell suspensions, characterized by rapid cell proliferation, sustained embryogenic potential, and synchronized somatic embryo production, have great potential for biotechnological applications such as plant transformation, genome editing or protoplast isolation, culture, and fusion. All these characteristics were found in gentian embryogenic cell suspension cultures developed by Mikuła and Rybczyński in 1994 (Mikuła & Rybczyński, 1994). This system has been successfully applied to basic biotechnological research by this scientific team. Many years of experience in SE of gentians have resulted in the description of this process 20 years later in ferns (Mikuła, Pożoga, Tomiczak, & Rybczyński, 2015). The tree fern Cyathea delgadii experimental protocol has provided a unique and simple system for cytological and physiological analyses of early SE events in single somatic cells. It also allows the factors stimulating the development of somatic embryo-derived sporophytes and the production of secondary metabolites (Tomaszewicz et al., 2022).
Research on SE in conifers were initiated in the 1990s to enable their efficient micropropagation for forestry and horticultural purposes at the Institute of Dendrology, Polish Academy of Sciences in Kórnik (ID PAS) (Żytkowiak, 1995). SE-based regeneration systems were developed for calluses derived from mature or immature zygotic embryos of Larix, Picea, and Abies under the leadership of Krystyna Bojarczuk. Initially, research was focused on developing improved protocols for micropropagation and embryogenic tissue cryostorage (Mikuła et al., in press). With the development of more modern research methods in biochemical and molecular analyses, there is constant knowledge expansion about the mechanisms regulating the development of somatic embryos of this group of plants under in vitro culture conditions.
The Model Plants
Among the various plant species for which SE has been described, model plants, such as A. thaliana and Medicago spp., have contributed the most to understanding the process. In Poland, SE research on A. thaliana was conducted in the late 1990s by Małgorzata Gaj at the Department of Genetics, Silesian University in Katowice (Gaj, 2001). The system established for culture of immature zygotic embryos as explants offers a simple, rapid, and effective method to regenerate Arabidopsis plants via direct SE (DSE). Since its first publication in 2001 (Gaj, 2001), the DSE method of Arabidopsis has been successfully applied in SE studies by many research groups worldwide. It is recommended as a model system to reveal the molecular determinants of embryogenic reprogramming in plant somatic cells.
Studies on SE in model plants developed in Poland also involve research on the embryogenic culture of M. sativa implemented in the early nineties of the twentieth century at the University of Szczecin. The first studies on SE in this species aimed to determine whether the action of endogenous ethylene is required for callus growth, induction of SE, and somatic embryo maturation (Kępczyński et al., 1992). Other papers published initially under the direction of Jan Kępczyński at the Department of Plant Physiology (Kepczynski & Florek, 1997) and later under the direction of Ewa Kępczyńska at the Department of Plant Biotechnology (Ruduś et al., 2001), presented a long-term study on the SE of M. sativa and another species of great value in genomic studies, M. truncatula (Igielski & Kępczyńska, 2017). The SE induction systems of model plants established by Polish groups are still being studied with a broad spectrum of experimental approaches and various genomic tools. In particular, research in this area aims to characterize SE at the transcriptomic, proteomic, chromatin, physiological, and cytological levels. The results obtained in Polish laboratories contributed to worldwide achievements in identifying genetic and epigenetic elements of the complex SE regulatory network controlling embryogenic induction in plant somatic cells.
. Embryogenic Cell Suspensions – A Milestone in Biotechnology of Gentiana spp.
Efficient cell suspension culture systems, established for gentians, allowed for in-depth research on (i) the process of SE and its extensive documentation, (ii) development of the basis of plant tissue cryopreservation, (iii) exploring cell reactions to dehydration stress, (iv) Agrobacterium-mediated transformation, protoplast isolation and their further direct transformation via electroporation, and (v) somatic hybridization via protoplast electrofusion. However, research over two decades has shown that the suspension culture system also has some limitations that should be examined critically before considering its use for different plant species. The exploitation of embryogenic suspension cultures is shown in Figure 1.
Figure 1
Scheme illustrating the use of embryogenic cell suspension cultures in the study and improvement of species belonging to the genus Gentiana. PEM – proembryogenic mass.

Cyclical Development of Proembryogenic Masses and Somatic Embryos in Suspension Cultures of Gentiana spp.
Studies on SE in gentians began in 1994 and resulted in the description of SE for G. cruciata and G. tibetica in two culture systems (i.e., on agar and in liquid medium) 2 years later (Mikuła et al., 1996). The embryogenic cell suspension system developed for these species was later applied to other gentians, namely, G. pannonica (Mikuła et al., 2002), G. kurroo (Fiuk & Rybczyński, 2008), and recently G. capitata and G. decumbens (Tomiczak & Markowski, 2021). Light, transmission (TEM), and scanning electron microscopy (SEM) have provided evidence of the single-cell origin of somatic embryos (Mikuła et al., 2001). Moreover, the cyclical nature of developing a proembryogenic mass (PEM) in G. pannonica and G. cruciata cell suspensions, which did not occur in G. tibetica culture, was later documented. This cyclical characteristic consisted of periodical (2-week long in a 6-week cycle) loss of regeneration ability of cell suspensions. In liquid medium supplemented with auxins and cytokinins somatic embryos cyclically developed from PEMs, until they reached globular stage. Subsequently, the embryo epidermis began proliferating, which led to the disintegration of embryo structures and the formation of a fine-aggregate suspension (Mikuła et al., 2002). The changes in the PEM of G. tibetica suspension culture took a different course. Following intense cell divisions, the globular embryo epidermis creates a starch zone consisting of cells rich in starch. Then, actively dividing outer cells formed the meristematic zone, in which some embryogenic cells evolved into secondary somatic embryos. All the above-described stages of PEM development occurred simultaneously in the culture. This disorganization of embryo structures and secondary induction of subsequent generations of embryogenic cells allow for the long-term maintenance of the embryogenic potential of gentian suspension cultures (Mikuła, Tomiczak, et al., 2015).
Mass Somatic Embryo Production and Aging of Suspension Cultures
Young gentian cell suspensions produced, depending on the species, 257 to more than 800 somatic embryos from 100 mg of PEM (Table 1). Many years of research have shown that with continuous culture there was a gradual reduction in SE effectiveness. It was completely inhibited in G. cruciata and G. pannonica after more than 3 years, after more than 6 years in G. kurroo, and after more than 13 years in G. tibetica. In addition, inhibition of embryogenic potential and aging of cell suspensions was also manifested by (i) enhanced dynamics of biomass growth (Mikuła, Tomiczak, et al., 2015), (ii) increased participation of the fine fraction of cell aggregates, (iii) an extended period – from 3 to 12 weeks needed to obtain the first somatic embryos on the regenerative medium, (iv) intensified morphological disturbances in the development of somatic embryos, and (v) gradual loss of their ability to convert into plants.
Table 1
Efficiency of somatic embryogenesis in cell suspension cultures of Gentiana spp. over many years of research.
| Age of culture | Number of somatic embryos per 100 mg tissue | |||
|---|---|---|---|---|
| G. cruciata | G. tibetica | G. kurroo | G. pannonica | |
| 2 months | - | - | 813(1) | - |
| 2 years | 226(2) | - | - | - |
| 3 years | 257(3) | 634(3) | - | 570(4) |
| 3.5 years | 18(5) | - | - | 0(6) |
| 6 years | - | 10–58(7) | ||
| 11 years | 266(2) | |||
| 12.5 years | 24(6) | |||
Preservation of Embryogenic Capacity at Liquid Nitrogen Temperature
Maintaining the embryogenic capacity of gentian cultures has led to the development of a universal cryopreservation technique for PEM for routine use. This procedure ensured an average survival of cell aggregates from 68% to 100% and facilitated simple and rapid recovery of embryogenic suspension cultures and increased somatic embryo production (Mikuła et al., 2008; Mikuła, Tomiczak, & Rybczyński, 2011; Mikuła, Tomiczak, Wójcik, & Rybczyński, 2011; Tomiczak & Markowski, 2021). Detailed research revealed that the embryogenic potential rose after exposure of the plant cells to long-lasting and gradually increasing osmotic stress during the pre-freezing procedures, which is an integral part of the encapsulation/dehydration technique. Moreover, freezing itself led to the death of strongly vacuolated cells and increased the embryogenic cell population (Mikuła, Tykarska, & Kuraś, 2005). Irrespective of the duration of cryostorage, the morphological uniformity of cell suspensions (Mikuła, Fiuk, & Rybczyński, 2005) and the genetic uniformity of regenerated plantlets were supported (Mikuła, Tomiczak, & Rybczyński, 2011; Mikuła, Tomiczak, Wójcik, & Rybczyński, 2011). The high viability of the tissues was maintained over 15 years of monitoring.
Embryogenic Cell Suspensions in Agrobacterium-Mediated Transformation of Gentians
Studies on the improvement and modification of gentian genomes could have been undertaken with a long-term and efficient source of highly embryogenic cell aggregates with established and universal protocols for plant regeneration for a few species. Indirect genetic transformation of G. cruciata and G. kurroo cell aggregates was conducted via their co-culture with bacterial cells of strain C58C1 of Agrobacterium tumefaciens carrying the nptII and uidA genes (Rybczyński & Wójcik, 2019). Long-lasting co-culture (24 or 48 hr), triple washing, weekly treatment with timentin-supplemented medium, and 3-month-long selection pressure on medium with kanamycin, reduced the embryogenic potential of G. cruciata cell suspension almost 14 times. However, 23 genetically modified somatic embryos were regenerated, and half were successfully converted into plants. It was the first report of Agrobacterium-mediated transformation of gentian cell suspension with the regeneration of transformants via SE.
High Embryogenic Potential of Gentian Cell Suspensions Enables Efficient Plant Regeneration From Their Protoplasts
Gentian embryogenic cell suspensions also appear to be an efficient source of protoplasts. In cultures of protoplasts isolated from young, freshly established cell suspensions of G. kurroo with very high morphogenic potential, regeneration via direct SE (i.e., formation of embryos directly from protoplast-derived cells) has been observed (Fiuk & Rybczyński, 2007). Direct SE did not occur in experiments employing older cell suspensions; however, the propensity of the cultured cells for somatic embryo formation was so strong that the media intended for plant regeneration and callus induction and proliferation triggered this process.
Another unique ability of the embryogenic characteristics of gentian suspension cells and protoplasts is their transfer to interspecific hybrid cells via protoplast electrofusion. Despite the recalcitrance of G. cruciata leaf mesophyll protoplasts (Tomiczak et al., 2016), hybrid cells formed after their fusion with cell suspension-derived protoplasts of G. kurroo were capable of SE, enabling the regeneration of 87 somatic hybrid plants G. kurroo (+) G. cruciata (Tomiczak et al., 2015, 2017). A similar situation occurred when low-regenerating mesophyll protoplasts of G. tibetica (Tomiczak et al., 2016) were electrofused with cell suspension-derived protoplasts of G. cruciata, yielding 82 somatic hybrids between these species (Tomiczak, 2020; Tomiczak et al., 2015). Despite several attempts at protoplast fusion worldwide, these are the only somatic hybrids within the genus Gentiana to date.
The above protoplast-to-plant system, which produces numerous regenerants, was also employed for sophisticated projects to modify the gentian genome via protoplast electroporation and direct transformation with a DNA plasmid. As a result of experiments using cell suspension-derived protoplasts of G. kurroo and the pBI plasmid carrying nptII and bar genes, nine transgenic plants were regenerated via SE on a selection medium supplemented with kanamycin (Wójcik & Rybczyński, 2015). This is the only report of the gentian protoplast transformation worldwide.
. The Tree Fern Cyathea delgadii as a Unique Experimental Model System for the Study of Somatic Embryogenesis
The tree fern C. delgadii appears to be the most valuable object in SE research. The results obtained at the Department of Plant Conservation Biology of PAS BG-CBDC using an experimental system for this species suggest more differences between somatic and zygotic embryogenesis than we thought.
Genesis of Research on Somatic Embryogenesis of Cyathea delgadii
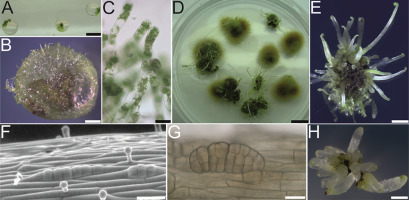
Scientific discoveries are often made accidentally. During the work on the cryopreservation of fern gametophytes in 2011, special attention was paid to the preculture stage (Mikuła, Makowski, et al., 2011). Light-free culture conditions were applied to limit the gametophyte proliferation encapsulated in alginate beads (Figure 2A). In the case of C. delgadii this led to the mass production of filamentous gametophytes (Figure 2B,C) that outgrew all beads after 6 months of culture (Figure 2D). Owing to this proliferation, numerous etiolated somatic embryos and then sporophytes were formed on the surface of the callus tissue (Figure 2D). In the dark, the petioles elongated rapidly, but the leaf blades remained at the crozier stage (Figure 2E).
By 2015, apart from two reports on SE in Lycopodiophyta (Atmane et al., 2000; Szypuła et al., 2005), there were no more published works on SE in seed-free vascular plants. One of the reviewers of the first paper describing this phenomenon in ferns (Mikuła, Pożoga, Tomiczak, & Rybczyński, 2015) wrote: “I am surprised that this capability has never been reported in monilophytes.” However, the most surprising finding was the simplicity of this process. The experimental system of SE in C. delgadii is characterized by (i) hormone-free induction medium, (ii) epidermal and unicellular origin of somatic embryos (Figure 2F,G), (iii) a short induction period (8 days), and (iv) high repeatability and effectiveness, that is, an average of 20 embryos per explant measuring only 2.5 mm in length (Figure 2H) (Mikuła, Pożoga, Grzyb, & Rybczyński, 2015; Mikuła, Pożoga, Tomiczak, & Rybczyński, 2015).
Figure 2
Somatic embryogenesis (SE) induction in Cyathea delgadii. (A–E) Spontaneous production of somatic embryos in the culture of encapsulated gametophytes maintained in darkness. (F–H) Production of somatic embryos from etiolated sporophytes conducted in darkness. (A) Gametophytes embedded in alginate beads. (B,C) Filamentous gametophyte proliferation, fourth month of culture. (D) General appearance of the culture after 6 months. (E) Numerous somatic embryo-derived sporophytes obtained by indirect SE after 6 months of culture. (F) Epidermal cells of stipe explant after several divisions perpendicular to the axis of the explant on the tenth day of culture. (G) Somatic embryo with visible segmental structure after 21 days of culture. (H) Juvenile sporophytes obtained from single stipe explants after 2 months of culture. Scale bars: (A) 5 mm; (E) 2 mm; (B,D,H) 1 mm; (C,F,G) 50 µm.
Experimental System of Cyathea delgadii
An experimental system for direct SE described in C. delgadii allows the study of its early phases, which are linked strongly to the response of the cells of the initial explant, that is, dedifferentiation, expression of totipotency, and the early stage of somatic embryo development.
Acquisition of Embryogenic Competence
Plant somatic cells are naturally nonembryogenic. In most spermatophytes, a somatic-to-embryogenic transition is induced on media supplemented with growth regulators in the presence of certain amino acids or stresses (Thorpe, 1995). Using the tree fern C. delgadii revealed that the application of typical exogenous factors may be unnecessary and can be replaced successfully by the long-term etiolation of donor plants (Grzyb et al., 2017). The content of the endogenous sugars and phytohormones and their ratios established by the etiolation of donor plants make their cells competent for embryogenesis (Grzyb et al., 2017). The stage of somatic cell reprogramming into totipotent cells is easier to control and describe using this approach. The short period required for the expression of totipotency in cultured explants (approximately 8 days after excision) and the unnoticeable structural changes (only the enhanced starch accumulation was visible) (Domżalska et al., 2017) may suggest that some somatic cells already acquired embryogenic competency in the donor plants. Similarly, the aging of plants grown in the dark for longer than 5 months releases this ability without needing exogenous hormone application (Mikuła, Pożoga, Tomiczak, & Rybczyński, 2015).
Cell Size Versus Capacity for Somatic Embryogenesis
Few studies have reported the morphological and structural characteristics of somatic cells and their possible relationship with embryogenic pathway initiation. Most studies present embryogenic cells that differentiate within the peripheral meristematic zone of the callus (Canhoto et al., 1996; Fransz & Schel, 1991; Mikuła et al., 2004; Verdeil et al., 2001). These small cells have dense cytoplasm, prominent nuclei, and small vacuoles. The experimental system of C. delgadii changed the previous view of cells capable of somatic embryo initiation. Focusing on the explant cells (instead of callus), it was shown that their size and length/width ratio did not determine embryogenic ability. Somatic cells shorter than 80 µm and longer than 400 µm produced embryos via direct SE (Mikuła et al., 2021).
Orientation of the First Divisions and the Embryo Polarity
Pteridophyte embryos develop in the relatively isolated archegonia of free-growing gametophytes. The orientation of the first division of the zygote is determined by the movement of macromolecules (including auxins) in haploid cells (Jayasekera & Bell, 1959). The first wall of the zygote tends to form transversely to the gametophyte plane and anterioposterior axis (Johnson & Renzaglia, 2008). After developing an eight-celled globular embryo, the initials of the first leaf, apical meristems of the root and shoot, and foot are defined early by discrete formative divisions. They develop into different embryonic organs depending on their location to the apical notch of the gametophyte and the archegonial neck (Johnson & Renzaglia, 2008). In SE of C. delgadii, the pattern of cell division initiating embryo formation is completely different from that in zygotic embryogenesis. This process is initiated by several divisions of epidermal cells that are perpendicular to the axis of the explant, leading to the formation of a linear somatic embryo (Mikuła, Pożoga, Tomiczak, & Rybczyński, 2015). This difference did not prevent normal embryonic development. A study conducted on C. delgadii provided evidence that the first cell divisions do not necessarily reflect those occurring during zygotic embryogenesis, as researchers often expect in the SE of seed plants.
Intercellular Communication
Isolation of somatic cells as a trigger for embryogenesis has been postulated for a long time (Aoshima, 2005; Verdeil et al., 2001). However, until research started on C. delgadii, the role of symplastic communication during somatic embryo formation was evaluated only in A. thaliana (Godel-Jedrychowska et al., 2020; Kurczyńska et al., 2007). Moreover, because of the difficulty in analyzing the earliest stages of SE, cell-to-cell communication during the embryogenic transition is poorly understood. The epidermal and unicellular origins of somatic embryos in C. delgadii were advantageous for assessing intercellular communication. This system can easily track it from the induction phase through the first embryogenic division to the formation of embryo segments from which organs differentiate. Current research has shown that the course of SE in C. delgadii depends on cytoplasmic connectivity between the cells of the initial explant and the cells of the somatic embryo (Grzyb et al., 2020). These studies also confirmed the occurrence of isolation during the induction of embryogenesis and differentiation of the embryo body. The mechanism regulating intercellular communication is yet to be elucidated. However, since the somatic embryo is isolated from the earliest stages of its development (three-cell structures), the pathway of somatic embryo formation can be changed from being multicellular to unicellular by sucrose treatment (Grzyb & Mikuła, 2019), which breaks the plasmodesmata connection, suggests its key role in fern SE. Current studies on cell wall reorganization may provide the answer to how communication is regulated, including changes in callose distribution at a very early stage of SE induction in C. delgadii.
Proteins Involved in Early Somatic Embryogenesis
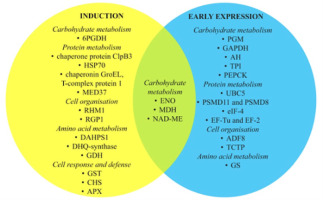
Tracking the molecular pathways involved in the SE of species without genetic data available is difficult. Although the genome of C. delgadii is not sequenced, differentially expressed proteins involved in early SE were analyzed (Figure 3) (Domżalska et al., 2017). The studies revealed that 13 of all proteins analyzed were strictly associated with the induction phase, while 12 were characteristic of the early phase of SE expression. Of these, several have been identified as potential proteomic markers for other species (Gulzar et al., 2020). This suggests that the SE regulation pathways in ferns may be shared with those in spermatophytes.
Figure 3
Differentially expressed proteins during induction and early expression of somatic embryogenesis of Cyathea delgadii categorized by biological function. ENO – enolase; MDH – malate dehydrogenase; NAD ME – NADP-dependent malic enzyme; 6PGDH – 6-phosphogluconate dehydrogenase, decarboxylating; PGM – 2,3-bisphosphoglycerate-independent phosphoglycerate mutase; GAPDH – glyceraldehyde-3-phosphate dehydrogenase; AH – aconitate dehydrogenase; TPI – triosephosphate isomerase; ALD – fructose-bisphosphate aldolase; PEPCK – phosphoenolpyruvate carboxykinase; UBC5 – ubiquitin-conjugating enzyme E2 5; HSP70 – heat shock proteins; PSMD11 and PSMD8 – 26S proteasome non-ATPase regulatory subunit 11 and subunit 8A; eIF-4 – eucaryotic translation initiation factor 4; MED37 – mediator of RNA polymerase II transcription subunit 37; EF-Tu and EF-2 – elongation factors; RHM1 – UDP-4-keto-l-rhamnose-reductase RHM1; RGP1 – UDP-arabinose mutase; ADF8 – actin-depolymerizing factor 8; TCTP – translationally controlled tumor protein; DAHPS1 – phospho-2-dehydro-3-deoxyheptonate aldolase 1; GS – glutamine synthetase; DHQ-synthase – 3-dehydroquinate synthase; GDH – glutamate dehydrogenase; GST – glutathione-S-transferase; CHS – chalcone synthetase; APX – l-ascorbate peroxidase.
. Conifer Somatic Embryogenesis – An Efficient Plant Regeneration System for Mass Propagation and Conservation
Somatic embryogenesis is the basic and most efficient method for the micropropagation of many conifer species. SE was first reported in 1985 for Picea abies L. Karst in this group of plants (Chalupa, 1985; Hakman et al., 1985) and Larix decidua Mill (Nagmani & Bonga, 1985). This research has initiated a period of intensive, multifaceted studies, both on the conditions that affect the efficiency of SE and the possibility of applying this technique in forestry and nursery practice in many research centers worldwide. In Poland, studies on the SE process of selected coniferous species were conducted at the Institute of Dendrology PAS, in the Institute of Forestry Research in Sękocin (currently terminated) and the University of Agriculture in Krakow. The first experiment was established in 1991 to propagate European larch (Larix decidua Mill.) in ID PAS. Three years later, an embryogenic tissue capable of multiplying under in vitro culture conditions, using mature zygotic embryos as explants, was obtained (Żytkowiak, 1995). Simultaneously, several experiments were conducted on Norway spruce, also using this type of explant. For initiating SE on mature embryos, seeds of Polish origin from 60–80-year-old excluded seed stands were collected. The study used cotyledons, hypocotyls, and buds of 1-month-old zygotic seedlings and somatic embryos, cotyledons, and hypocotyls of 1-month-old somatic seedlings. In the following years, studies were conducted on applying the micropropagation method based on SE of silver fir (Abies alba Mill.) and European larch of native origin. These studies obtained positive results from the material originating from the Polish seed plantations of Picea abies, P. omorika, P. pungens ‘Glauca,’ P. breweriana, A. alba, Pinus sylvestris, and the Danish populations of A. nordmanniana. It was possible to obtain somatic embryos and somatic seedlings for all the coniferous species tested (Hazubska-Przybył & Bojarczuk, 2008; Nawrot-Chorabik, 2009, 2015, 2016). Embryogenic calli, periodically subcultured to maintain embryogenic potential, were then used for cryopreservation. Efficient protocols for storing species such as P. abies and P. omorika in liquid nitrogen have been developed using this approach (Hazubska-Przybył et al., 2010, Hazubska-Przybył, Chmielarz, et al., 2013). In Poland, the cryostorage of conifer embryogenic tissues is a useful tool for reliable long-term maintenance of specific genotypes with higher yield potential and wood quality and better resistance to pests.
A Specific “Memory” of Subsequent Generations of Callus
The reproduction of coniferous species by SE is a complex, multistep process. Its final efficiency is linked closely to success at individual stages of embryo development. Therefore, in the first research stage, it is crucial to develop or adapt the methodology to the conditions in a particular laboratory and make the starting material used to initiate embryogenesis available to the researcher. For this reason, research in Polish laboratories has largely focused on improving micropropagation protocols (Hazubska-Przybył & Bojarczuk, 2008; Szczygieł et al., 2007). These studies indicate that the efficiency of SE for the tree species depends on many interacting factors, including (i) the origin of explants, (ii) their stage of development, (iii) the mineral composition of the media, (iv) the type and concentration of growth regulators added to the media, (v) the length of their application, and (vi) the conditions under which the cultures were grown. It has also been shown that SE efficiency could be improved by initiating the embryogenic calli of the first, second, and third generations on somatic embryos as explants (Hazubska-Przybył & Bojarczuk, 2008). This procedure improved somatic embryo production from embryogenic callus in subsequent generations due to a specific “memory.” Consequently, a greater number of embryos were obtained during maturation.
Effect of Genotype
The efficiency of SE in conifer species at particular stages is correlated strongly with the genotype from which embryogenic culture was obtained (Hazubska-Przybył & Bojarczuk, 2008). Norway spruce embryogenic tissues of different genotypes showed a differentiated response to light conditions during their in vitro multiplication (Latkowska et al., 2000). Similarly, a clear effect of genotype was observed in response to adding specific components such as sucrose or auxins to the culture medium during the induction of SE or abscisic acid (ABA) during embryo maturation (Hazubska-Przybył et al., 2016). ABA concentration and osmotic pressure significantly affected the production and maturation of Norway and Serbian spruce embryos and the content and pattern of starch accumulation in somatic embryos, consequently affecting their quality (Hazubska-Przybył et al., 2016).
Guaiacol Peroxidase as a Possible Biochemical Marker of Somatic Embryogenesis
Peroxidases belong to the group of oxidoreductases and catalyze the oxidation of hydrogen peroxide, thereby protecting plant cells from oxidative stress. They also participate in the metabolism of auxins and ethylene and the restoration of the cell wall; hence, they are referred to as possible “morphogenesis markers” (Kawano, 2003; Konieczny et al., 2008). Little is known about the activity of guaiacol peroxidase during the induction of SE and the further development of somatic embryos. Variable guaiacol peroxidase activity was observed during the SE of Eleutherococcus senticosus (Shohael et al., 2007) or Helianthus annuus (Konieczny et al., 2008). Biochemical analyses carried out by Hazubska-Przybył, Ratajczak, et al. (2013) demonstrated significant changes in guaiacol peroxidase activity at the induction and proliferation stages of embryogenic tissues in Norway and Serbian spruce. The lowest level of activity was observed in the initial explants (mature zygotic embryos). The activity was significantly higher in 8-week-old non-embryogenic and embryogenic calli, whereas at the tissue multiplication stage, the activity of this enzyme remained at a lower level.
On the other hand, a study on the influence of three synthetic auxins [2,4-dichlorophenoxyacetic acid (2,4-D), 1-naphthaleneacetic acid (NAA), and 4-amino-3,5,6-trichloropicolinic acid (picloram)] on individual stages of development in Norway and Serbian spruce somatic embryos demonstrated that guaiacol peroxidase activity was significantly dependent on the type of auxin added to the multiplication medium and was lower in the presence of NAA (Hazubska-Przybył et al., 2020). Recently, its activity was documented in liquid and semi-solid cultures of Norway spruce (Välimäki et al., 2021). Previously, Shohael et al. (2007) observed guaiacol peroxidase activity during the maturation of E. senticosus somatic embryos. Therefore, changes in the activity of this enzyme could indicate that it is involved in the regulation of embryogenesis, and it may be a biochemical marker of this process in both tested spruce species. Studies on the role of guaiacol peroxidase during SE will be continued to confirm the role of this enzyme in the whole process by determining the pattern of its activity in the studied spruce species.
Dual Cultures and Abiotic Stress
In recent years, an interesting study on the effect of stress conditions on the non-embryogenic calli of Scots pine was conducted (Nawrot-Chorabik et al., 2016). The subject of this study was the interactions between undifferentiated callus cells of host plants and pathogenic, endophytic, or saprophytic fungi. Studies conducted in dual cultures have shown that pine calli are susceptible to fungal virulence. A similar correlation was detected in other experiments using dual cultures of silver fir embryogenic tissues and three Heterobasidion species (Nawrot-Chorabik, 2014). This experimental system makes it possible to quickly assess the degree of threat from the pathogen and select plant genotypes that are more resistant to it. Embryogenic tissues obtained via SE enable the study of pathogenicity in the early stages of embryonic development. According to Woodward and Pearce (1988), defensive reactions of plants against pathogens are observed at this stage. Based on the tissue reaction to the pathogen, it is possible to determine the level of aggressiveness using these sensitive tissues. This would be particularly useful in assessing the pathogen resistance of elite embryogenic lines intended for cultivation in forest plantations. Stress caused by heavy metals such as lead, cadmium, and copper in Caucasian fir in vitro cultures was also investigated using embryogenic tissues (Nawrot-Chorabik, 2017). The main assumption of these studies was the acquisition of tolerance in embryogenic tissues exposed to this type of abiotic stress. Consequently, it would allow an understanding of issues related to the resistance of plant genotypes to heavy metals and facilitate the selection of genotypes resistant to such stress factors in the future, especially in forest management.
. Molecular Mechanism Controlling Somatic Embryogenesis Induction – A Lesson From a Model Plant Arabidopsis thaliana
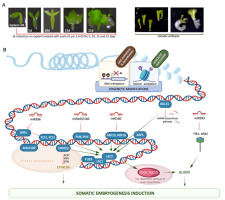
The unique developmental capacity of plants, manifested by SE, has been widely studied in different species for over 60 years. Insights into SE induction in A. thaliana, a model in plant genomics, have contributed significantly to our knowledge of embryogenic reprogramming of plant somatic cells at the molecular level (reviewed in Wójcik et al., 2020; Wójcikowska & Gaj, 2016; Wójcikowska et al., 2020). The establishment of an efficient SE induction method in this species at the Department of Genetics launched the research on molecular determinants of SE at the University of Silesia in Katowice in the late 1990s. In this method, immature zygotic embryos (IZEs) at the late cotyledonary stage of development cultured in vitro on an auxin medium with 2,4-D developed into somatic embryos within two weeks (Figure 4A) (Gaj, 2001, 2011). This process is highly efficient, and over 90% of explants in Col-0, an ecotype widely studied in plant genomics, produce somatic embryos that regenerate into plants on a hormone-free medium. Histological analysis showed a uni- and multicellular origin of somatic embryos, developing mainly via DSE from the adaxial side of the IZE cotyledons (Kurczyńska et al., 2007). This simple and efficient protocol for SE induction, with the versatile molecular tools and public resources of genomic data available for A. thaliana, initiated research on the embryogenic switch in plant somatic cells at the molecular level.
Figure 4
Auxin-induced somatic embryogenesis (SE) in the model plant Arabidopsis thaliana (A) contributed to the deciphering of the genetic (transcription factors) and epigenetic (miRNA, DNA methylation, Hac) components of the regulatory network controlling the embryogenic transition in plant somatic cells (B). The elements indicated in the SE network are described in the text.
Transcription Factors
The embryogenic switch in somatic cells, requires extensive reprogramming of the cell transcriptome, governed by regulatory proteins and transcription factors (TFs). TFs that repress or activate target gene transcription play crucial roles in controlling SE induction (reviewed in Nowak & Gaj, 2016b). Multi-parallel qRT-PCR analysis of almost 1,900 TF genes identified numerous (over 700) TFs with substantial changes in transcript accumulation in embryogenic cultures of A. thaliana (Gliwicka et al., 2013). Consistent with the critical role of phytohormones in SE induction, hundreds of SE-modulated TF genes have been annotated to mainly the phytohormone auxin-related processes. Functional analysis of several SE-deregulated TFs revealed their roles in the genetic pathways that control SE induction. Below is a brief description of the functions of TFs in the SE regulatory network identified in A. thaliana.
LEAFY COTYLEDON2 (LEC2) TF via interactions with other LEAFY COTYLEDON (LEC), that is, LEAFY COTYLEDON1 (LEC1) and FUSCA3 (FUS3) of the master regulatory role in plant embryogenesis, promotes the embryogenic transition of somatic cells by controlling the endogenous auxin content via the YUCCA (YUC)-dependent auxin biosynthesis pathway (Gaj et al., 2005; Ledwoń & Gaj, 2009; Wójcikowska et al., 2013; Wójcikowska & Gaj, 2015).
Different AUXIN RESPONSE FACTORs (ARFs) with core regulatory roles in the auxin signaling pathway contribute to auxin-induced SE (Wójcikowska & Gaj, 2017). More specifically, it is postulated that ARF10/ARF16, PHABULOSA (PHB) and PHAVOLUTA (PHV) of the class III HOMEODOMAIN LEUCINE ZIPPER (HD-ZIP III) TF family might control embryogenic transition, indirectly affecting LEC2 expression and modulating auxin biosynthesis in the explant tissue (Wójcik et al., 2017).
AGAMOUS-LIKE 15 (AGL15) of the MADS-box family of TFs, affects SE induction by regulating miR156. In this mechanism, AGL15 co-operates with TOPLESS co-repressors (TPL and TPR1-4), which are components of the SIN3/HDAC silencing complex, to repress miRNA biogenesis genes, including DICER-LIKE1 (DCL1), SEERATE, and HUA ENHANCER1 (Nowak et al., 2020).
ETHYLENE-RESPONSIVE TRANSCRIPTION FACTOR022 (ERF022) controls SE induction via ethylene-related pathways. The results of SE in A. thaliana suggest that ERF022 regulates the genes involved in ethylene biosynthesis (1-AMINO-CYCLOPROPANE-1-CARBOXYLATE SYNTHASE 7, ACS7) and signaling (ERF1 and ETHYLENE RESPONSE 1, ETR1). Moreover, regulatory relationships between ERF022 and LEC2, key regulators of auxin biosynthesis in SE, have been postulated (Nowak et al., 2015).
The function of bHLH109 in the BASIC HELIX–LOOP–HELIX (bHLH) transcription factors (TFs) in SE induction has been attributed to stress responses of a central position in plant embryogenic transition, including in A. thaliana (Gliwicka et al., 2013; Nowak & Gaj, 2016a). It is assumed that bHLH109 promotes the accumulation of the late embryogenesis abundant (LEA) protein (ECP63), which results in the enhanced tolerance of explant cells to stress and increases the embryogenic response (Nowak & Gaj, 2016a).
The role of AP2 (APETALA2) from the AP2/ERF (APETALA2/ETHYLENE RESPONSE FACTOR) family of TFs in controlling WUS (WUSCHEL) TF in SE induction. It was revealed that under the control of miR172, AP2 regulates WUS transcription, and the regulatory mechanism involves the AP2-mediated recruitment of the histone deacetylases HDA6/HDA19 to the target gene. The upstream regulatory elements of the miR172–AP2–WUS pathway might involve miR156-controlled SPL9/SPL10, which controls the level of mature miR172 in embryogenic culture (Nowak, Morończyk, et al., 2022).
MicroRNA (miRNAs)
miRNAs also play an essential role in fine-tuning the cell transcriptome during plant development. In support of the critical role of miRNAs in SE, a dcl1 mutant defective in the DCL1 gene, which plays an essential role in miRNA biogenesis, was unable to induce SE (Wójcik & Gaj, 2016). Thus, the identification of miRNAs and their targets controlling SE induction was performed. Extensive modulation of numerous miRNAs has been reported to accompany SE induction (Szyrajew et al., 2017). Several SE-modulated miRNAs have been functionally analyzed using different genomic approaches, including whole-mount in situ hybridization of sRNA (Wójcik et al., 2018; Wójcik, 2020). This analysis revealed the complex interactions of different miRNA-controlled modules in the auxin-induced mechanism of SE. Accordingly, it was demonstrated that the miR165/166 and miR160 contribute to the LEC2-controlled pathway of SE induction by targeting PHB/PHV and ARF10/ARF16, respectively (Wójcik et al., 2017). Moreover, miR393 was found to affect SE induction by modifying tissue sensitivity to auxin treatment by targeting the core components of the auxin signaling pathway, auxin receptor TRANSPORT INHIBITOR RESPONSE 1 (TIR1) and AUXIN SIGNALING F-BOX PROTEINS (AFB1 and AFB2) (Wójcik & Gaj, 2016). Auxin-related function in SE has also been implicated in miR396. miR396 might affect SE induction by targeting GROWTH-REGULATING FACTORS (GRFs) such as GRF1, GRF4, GRF7, GRF8, GRF9, and PLETHORA (PLT1 and PLT2) TFs (Szczygieł-Sommer & Gaj, 2019).
Epigenetic Modulators: DNA Methylation and Histone Acetylation
In cooperation with TFs and miRNAs, epigenetic modifications of DNA and chromatin substantially modulate gene expression, thus controlling transcriptome reprogramming associated with SE induction (reviewed in Wójcikowska et al., 2020). Thus, another study focused on the roles of DNA methylation and histone acetylation in SE induction. The study found that SE induction is associated with substantial global demethylation of DNA, accompanied by differential expression of genes encoding enzymes that methylate and demethylate DNA. Congruently, mutations in DNA methylase/demethylase genes substantially affected the SE response. Importantly, significant repression of the LEC2, LEC1, and BABY BOOM (BBM) genes in the explants incapable of SE induction upon treatment with 5-azacitidine (5-AzaC) of the DNA demethylation activity were indicated (Grzybkowska et al., 2018). Thus, it can be assumed that DNA methylation regulates SE induction by controlling the SE-involved genes. Changes in DNA methylation in the promoters and gene body sequences of SE-essential TF genes were analyzed to verify this hypothesis. The results showed that auxin treatment substantially affected the expression and methylation patterns of master SE regulators (LEC1, LEC2, BBM, WUS, and AGL15), providing experimental evidence of the contribution of DNA methylation to epigenetic regulation of genes in SE. It is noteworthy that SE-efficient auxin treatment (5 µM 2,4-D) was associated with a specific level of DNA methylation in the promoter and gene body sequences of the SE-involved TFs (Grzybkowska et al., 2018).
In contrast to DNA methylation, the role of chromatin modification in SE induction remains poorly understood. Previous research has provided evidence on the essential contribution of histone acetylation (Hac) to SE regulation. It was indicated that trichostatin A (TSA), a chemical inhibitor of histone deacetylases, induced SE in explants cultured on an auxin-free medium (Wójcikowska et al., 2018). In addition, SE-induced explants showed substantial changes in Hac at global and gene-specific levels (Morończyk et al., 2022). Thus, identifying genes associated with Hac-regulated expression in SE was performed. RNAseq analysis revealed that numerous candidate genes related to metabolism, signaling, polar transport of auxin, and stress responses were highly deregulated in response to TSA treatment. Thus, Hac is assumed to control these genes in SE (Wójcikowska et al., 2022). This assumption was verified using the ChIP method, which demonstrated that Hac, mediated by histone acetyltransferases and deacetylases, including HAG1/GCN5 and HDAC19, might control TFs including LEC1, LEC2, and BBM with essential roles in SE. More specifically, it indicates the role of HDAC19 in the histone acetylation-mediated regulation of LEC2 in SE. The results on DNA methylation and histone acetylation significantly improved our knowledge of epigenetic mechanisms controlling embryogenic reprogramming of plant somatic cells.
Contribution of Studies on Somatic Embryogenesis in Arabidopsis thaliana to Cereal Biotechnology
The SE-promoting effect of TSA in A. thaliana may be implemented to improve in vitro propagation of other plant species. TSA treatment of barley (Hordeum vulgare L.) explants might increase plant regeneration efficiency in some recalcitrant genotypes, including scarlet and krona. Moreover, barley homologs of genes controlling SE induction in A. thaliana are differentially regulated in barley calli that regenerated plants (Nowak, Wójcikowska, et al., 2022). These findings suggest some similarities in the regulatory mechanisms controlling in vitro regeneration in A. thaliana and other plants, including monocots. Thus, the knowledge gained from studies on SE in this model species might improve the regeneration capacity of in vitro recalcitrant cereals such as H. vulgare.
. Medicago spp. as a Model Legume Plants for Studying In Vitro Somatic Embryogenesis
Somatic embryogenesis plays an important role in plant regeneration and the large-scale propagation of several agronomically important species, including representatives of the Fabaceae family, such as M. sativa and M. truncatula. It also offers an in vitro experimental system for studying the physiological and molecular basis of cell differentiation and embryonic development. SE fundamental phases are characterized at the morphological level by the induction of proembryonic structures (induction phase, IP), followed by somatic embryo formation (differentiation phase, DP) and maturation, leading to plant regeneration. The embryogenic properties of Medicago sp. and other plants are correlated mainly with the genotype of the donor plants. Among external factors, phytohormones, mainly auxin and cytokinin, commonly used in the form of their synthetic analogs (2,4-D and kinetin, respectively), play the most crucial role in SE. The most successful understanding of the mechanisms involved in SE induction and expression in agronomically important plants has been accomplished using model plant species, such as carrot, white spruce, and alfalfa. It is necessary to understand signal transduction processes and their relationship with the presence of growth regulators in the medium and their endogenous levels in plant explants. Investigations of the role of phytohormones in different phases of indirect SE in M. sativa ‘Rangelander’ was developed two decades ago at the Department of Plant Biotechnology of the University of Szczecin. These studies were carried out in two regenerative systems and extended using the non-embryogenic genotype (M9) and embryogenic variant (M9-10a) of M. truncatula ‘Jemalong’ (Figure 5).
Figure 5
The scheme of regenerative systems in Medicago spp. developed for (A) M. sativa ‘Rangelander’ and (B) M. truncatula ‘Jemalong.’

The regenerative System I was based on the proposition of Meijer and Brown (1987), whereas System II used the method of McKersie et al. (1989). Both systems can obtain somatic embryos, but they differ in the number and duration of the phases distinguished by their course and the type of media used (Figure 5A). The first two-stage system with a distinguished induction and differentiation phase allows for the relatively quick and uncomplicated acquisition of somatic embryos from petiole-derived calli, although most of them reach the globular and heart-shaped stages, and only a few develop into the cotyledon stage. This system allowed accurate determination of the participation of each of the phytohormones of interest in the induction of embryogenicity and initiation of somatic embryo formation. However, apart from the number of embryos produced, it is difficult to trace the stages of embryo development accurately. A longer, more labor-intensive System II was more appropriate for this purpose. The use of both systems facilitated a detailed and thorough examination of the role of phytohormones in distinct phases of SE.
Hormonal Regulation of Somatic Embryogenesis
Using M. sativa ‘Rangelander’ and M. truncatula ‘Jemalong’ systems, new data on the participation of ethylene, gibberellins (GAs), ABA, jasmonic acid (JA) and methyl jasmonate (MeJA) were provided by: (i) the application of all hormones as mentioned earlier (except ethylene) in the distinct SE phases, (ii) the application of inhibitors of biosynthesis of GAs (ancymidol, paclobutrazole), ABA (fluridone), jasmonates (ibuprofen, indoprofen, antipyrine) and ethylene [aminoethoxyvinylglycine (AVG), salicylic acid (SA)], and (iii) the application of inhibitors of ethylene action [2,5-norbornadiene (NBD), methylcyclopropene (1-MCP)] simultaneously with a determination of the levels of endogenous phytohormones [ethylene, ABA, JA, indole-3-acetic acid (IAA)] and their precursors, e.g., 1-aminocyclopropane-1-carboxylic acid (ACC) for ethylene and 12-oxophytodienoic acid (OPDA) for jasmonates. Exogenous ABA and jasmonates (JA/MeJA) inhibit callus growth, the proliferation of cell suspensions, induction of embryogenicity, and production of somatic embryos in M. sativa (Ruduś et al., 2001, 2006). ABA is more potent than MeJA as a somatic embryo germination and conversion inhibitor. In contrast to ABA, MeJA did not significantly influence the development of somatic embryos when applied to the differentiation medium. It has been shown that ABA, used routinely as an inducer of somatic embryo maturation, could not be replaced by MeJA (Ruduś et al., 2006). Moreover, new data on stress-related phytohormones (ABA, jasmonates, ethylene) are synthesized in tissues of M. sativa L. during an indirect SE, and the biosynthetic activity changes in distinct phases of this process were provided (Kępczyńska et al., 2009a; Ruduś et al., 2005, 2009). Any alteration in ABA, JA, and ethylene synthesis during distinct phases of SE proved unfavorable for subsequent somatic embryo production, suggesting that the levels of these hormones are optimal for sustaining an appropriate course of this developmental process. Surprisingly, a low level of IAA has been detected in primary explants, embryogenic suspensions, and embryos at all developmental stages (Ruduś et al., 2009). Furthermore, in the case of ethylene, it has been demonstrated that not only biosynthesis but also action is involved in the control of individual phases of SE in M. sativa (Kępczyńska & Zielińska, 2011; Kępczyński et al., 1992). Disrupting both processes by inhibitors during the proliferation and differentiation phases reduces embryo regeneration (Kępczyńska & Zielińska, 2011). ABA and MeJA have been shown to exert inhibitory effects on somatic embryo formation by modifying ethylene production (Kępczyńska et al., 2009b). Moreover, for the first time, the involvement of endogenous ethylene in the regulation of M. sativa embryo germination and conversion was demonstrated by controlling the activity of α-amylase, an enzyme responsible for starch hydrolysis (Kępczyńska & Zielińska, 2013).
There are contrasting reports on the contribution of GAs, classical growth stimulants, in SE. In M. sativa and M. truncatula embryogenic lines, endogenous GAs play a stimulatory role during the induction and differentiation of somatic embryos (Igielski & Kępczyńska, 2017; Ruduś et al., 2001). However, exogenous application of GA3 to the induction medium had a different effect on the development of somatic embryos than when applied to the differentiation medium. This phytohormone inhibited M. sativa and M. truncatula callus growth but inhibited subsequent embryo production only in M. truncatula. These results suggest that the level of endogenous GAs may be sufficient for callus growth and embryo formation, depending on the tissue type. It has also been shown that exogenous GA3 can be used to improve the regeneration of somatic embryos because it accelerates starch hydrolysis by increasing α-amylase activity (Kępczyńska & Zielińska, 2006). Using the non-embryogenic genotype (M9) and embryogenic variant (M9-10a) of M. truncatula (Figure 5B), earlier data on the involvement of GAs and ABA in the induction of SE, obtained with the use of M. sativa, were confirmed (Igielski & Kępczyńska, 2017). It was shown for the first time that during the induction phase, when leaf explants of both lines form calluses, all the bioactive GAs from two different biosynthetic pathways, GA4, GA7 and GA1, GA5, GA3, and GA6 were present, but mainly biosynthesis of bioactive GA3 accompanies SE induction in the M9-10a genotype. Of the 20 fully annotated M. truncatula orthologous genes encoding the enzymes catalyzing all reactions of GAs synthesis, only the expression of three was specific to embryogenic tissues and reflected the changes in the contents of the substrates GA53 and GA19 and bioactive GA3. Exogenous application of GA3 during the induction phase inhibited not only M9-10a callus growth but also subsequent embryo production on differentiation medium and decreased expression of MtBBM, detected only in the embryogenic line. This is the first study to show that two genotypes (M9 and M9-10a) belonging to the same species and cultivar differ in their ABA, GAs, and IAA metabolism (Kępczyńska & Orłowska, 2021). The induction of embryo formation was related to a lower ABA content compared with the content of IAA and the total content of bioactive GAs. Regardless of the species, primary explants (M. sativa – petioles; M. truncatula – leaves) have a higher level of ABA than IAA (Kępczyńska & Orłowska, 2021; Ruduś et al., 2009).
Genetic and Epigenetic Regulation of Somatic Embryogenesis
Studies on Medicago spp. have provided new data regarding the involvement of LEAFY COTYLEDON 1-LIKE (L1L), LEC1, SHOOT MERISTEMLESS (STM), and WUS genes in processes leading to the formation of embryogenic cells (Orłowska et al., 2017; Orłowska & Kepczyńska, 2018). L1L, LEC1, and STM may be used as gene markers for SE in M. truncatula as their expression was observed only in the embryogenic genotype. For the first time, new epigenetic data related to the involvement of genes encoding proteins from the polycomb repressive complex 2 (PRC2), PRC1, and Thritorax complexes during the induction phase of SE have been shown (Orłowska et al., 2017; Orłowska & Kępczyńska, 2018, 2020b). The lower expression levels of all the PRC1 genes and vernalization 2 (VRN2), histone-binding protein MSI1, and FIE from the PRC2 complex in primary explants of the embryogenic genotype than in the non-embryogenic line probably trigger processes leading to the development of embryogenic cells. This is perhaps related to the more than twofold higher ABA content in primary explants of a non-embryogenic line than that found in the embryogenic line (Kępczyńska & Orłowska, 2021).
It was shown that there was a higher ratio of the content of growth stimulants (GAs, IAA) to the growth inhibitor (ABA) responsible for the induction of SE in M. truncatula. In addition, during the induction phase, the appropriate accumulation of reactive oxygen species (ROS), including the superoxide anion ensured by the proper 2,4-D concentration in the medium, is necessary for the induction and regulation of SE at the epigenetic and transcriptional levels (Orłowska & Kępczyńska, 2020a, 2020b).
. Final Remarks
Research conducted by Polish scientists has made considerable contributions to global science by improving knowledge in the field.
The discovery and description of plant regeneration via SE in gentians, as well as the implementation of a stable suspension culture system maintaining the embryogenic potential for a long time, was a breakthrough in research and laid the foundation for a broad biotechnological application. It has been demonstrated that the organs, tissues, cells, and protoplasts of gentians possess morphogenetic capabilities of an embryogenic nature. This potential allows for manipulating the genome of these plants to acquire a new biological value.
Studies on the tree fern C. delgadii have provided an excellent model system for understanding various events occurring during the transition of a somatic cell into an embryogenic cell and early embryo development, especially in ferns. This area of research is a blank slate. The availability of fern SE will certainly improve our understanding of the evolution of embryogenic development in higher plants, including the asexual formation of embryos.
Research on conifers has shown that SE efficiency is closely related to the species and genotype of the obtained embryogenic lines. The developed cryopreservation procedures allow long-term storage of elite cell lines of Polish origin in the forest gene bank. Studies on guaiacol peroxidase have revealed that this enzyme can be used as a biochemical marker of SE. While studying the effect of dual cultures and heavy metals, it was found that conifer tissues respond differently to fungal pathogens and stress factors. This will allow the selection of genotypes most tolerant to adverse environmental conditions and their introduction into forest management.
Studies on A. thaliana have revealed that genetic and epigenetic factors, including TFs, miRNAs, DNA methylation, and histone acetylation, play a central role in erasing the existing cell identity and switching on the embryogenic developmental pathway in already differentiated somatic cells of plants. The versatile regulatory interactions between the components of the SE regulatory network harmoniously orchestrate the embryogenic transcriptome in response to auxin treatment (Figure 4B). However, numerous questions regarding SE regulatory mechanisms remain open. Future studies will decipher other essential players in the SE-involved regulatory pathways.
Research conducted on Medicago ssp. indicated that competence for the induction of SE is highly correlated with the genotype, as exemplified by M. truncatula ‘Jemalong’ embryogenic variant M9-10a is considered a crop model for the study of legume SE. This line was derived directly from the non-embryogenic genotype M9, making it possible to compare when SE is “switched on” or “switched off.” Knowledge of changes in hormone levels and their metabolites throughout SE can be applied to implement research on the crosstalk between the signaling pathways that influence phytohormones.


