. Introduction
The development of different technologies and the description of various protocols to produce homozygous plants has had a substantial impact on agricultural systems. The creation of haploid plants and doubled haploids (DHs) offers a particularly appealing biotechnological tool since, nowadays, numerous agronomically significant crop breeding programs include these biotechnologies as a fundamental component of progress. While there are several ways to produce haploids and DHs, the most popular and efficient ones are in vitro anther or isolated microspore cultures (Germanà, 2009). An alternate developmental pathway for microspores, known as androgenesis, involves their reprogramming and gametophytic development redirecting to a sporophytic pathway and embryo development. In nature, this means that spontaneous haploidization in which an embryo with a purely paternal genetic program arose in the embryo sac after the failure of the egg nucleus to participate in fertilization (Kiełkowska & Kiszczak, 2023). Today, the term “androgenesis” refers to male-derived haploids produced in vitro in the laboratory using a variety of techniques, including culture of anther, isolated microspores, or meiocyte-derived callus (Seguí-Simarro, 2010). The reduction of time and expense required for new cultivar development are among the main benefits of using DHs in breeding. Additionally, labor-intensive inbreeding is not required since desired genotypes are fixed in one generation. Potentially, androgenesis is the most efficient way to produce DH plants. However, several valuable species of various crops are resistant to this procedure, including rye (Secale cereale L.), maize (Zea mays L.), oat (Avena sativa L.) and potato (Solanum tuberosum ssp. tuberosum) (Kiviharju et al., 2017; Małuszyński et al., 2003). Even though developing embryo-like structures (ELS) and then androgenic embryos is by far the most popular method for creating DH in cereals, numerous factors leading to the reprogramming of microspores, followed by embryo development under in vitro culture optimal conditions must be included. Pretreatments such as cooling, heating, high humidity, water stress, anaerobic treatment, centrifugation, sucrose and nitrogen starvation, ethanol, electrostimulation, high-pH medium, and heavy metal treatment are the main factors influencing anther and microspore culture, which have been reviewed in detail by Shariatpanahi et al. (2006).
There are twenty-five annual plant species in the genus Avena L., including weeds, wild species, and agricultural crops. The primary farmed species, common oat (A. sativa L.), accounts for 90% of global oat output. Currently, just 1.5% of the land used for cereals is planted with oat and farmed on around 10 million hectares worldwide. Although it is mostly used for human consumption and animal feed, oat grain is also becoming more significant in the cosmetics and pharmaceutical sectors (Leszczyńska et al., 2023) or as a potential source of energy (Tobiasz-Salach et al., 2023). Oat is a less important crop economically and commercially than other cereals, but their health advantages have been proven, making them an interesting topic for genetic and breeding studies. However, the huge size and complexity of the oat genome are major obstacles to adopting common research methodologies, which has resulted in little progress in oat research (Gasparis, 2017). Even though more than 40 years have passed since Rines (1983) obtained the first oat plants by androgenesis, and despite advancements that have been made in the efficiency of techniques based on microspore embryogenesis in cereals, oat is still regarded as a resistant species in this process.
Morphological markers are particularly helpful in determining the optimal explant stage to be introduced in vitro in order to determine the appropriate microspore developmental stages for effective embryogenesis induction. Furthermore, understanding the morphological traits of the pistil, spikelet, and flower sizes in relation to the stages of the ovule, embryo sac, and pollen development has proven to be useful for molecular and cellular research (Koehler et al., 2023). The non-linear anther maturation caused by the design and shape of oat panicles, and therefore observation of anthers size carried out prior to in vitro culture, may help define the microspores’ developmental stage. A study by de Cesaro et al. (2009) confirmed that the developmental stage of microspores is contingent upon the anthers’ location in the inflorescence, which causes them to mature unevenly, rather than the genotype and age of the plant. It is, therefore, possible that the low efficiency of embryogenesis obtained in oat anthers culture may be partly explained by difficulties in adequately monitored stage of microspore development.
Several factors have been found to significantly affect the response of anther cultures in A. sativa, including the donor plants’ growth conditions, the temperature of the pre-and post-treatments (Kiviharju et al., 1998; Sidhu & Davies, 2009; Warchoł et al., 2019). In the study of Ślusarkiewicz-Jarzina and Ponitka (2007), oat panicles were cold-treated at 4 °C for a few days in an N6 medium (Chu et al., 1975) containing 2,4-dichlorophenoxyacetic acid (2,4-D). Sidhu and Davies (2009) used 4 °C temperature to induce oat androgenesis over a 6–9 weeks. Although pretreatment is often carried out at low temperatures, oat anther can also be subjected to heat shock conditions of 32 °C for days (Kiviharju et al., 1998), for 24 hours (Warchoł et al., 2019) or for 24 hours at 35 °C after being placed on induction media (Rines et al., 1997).
Besides stress treatments, most of the investigations focused on culture media components, the media’s basic mineral salt levels (Ponitka & Ślusarkiewicz-Jarzina, 2009; Warchoł et al., 2019), carbon and nitrogen sources and concentrations (Kiviharju & Pehu, 1998), liquid versus solidified media (Ślusarkiewicz-Jarzina & Ponitka, 2007), different growth regulator compounds (Kiviharju & Tauriainen, 1999; Warchoł et al., 2019), and Cu2+, Zn2+, or Ag+ ion supplementation (Warchoł et al., 2021b). Salt concentrations in media for anther culture are often lower than those in micropropagation media (Murovec & Bohanec, 2012), although there is no set rule for this. To induce androgenesis in oat, common sources of macro- and microelements are used, such as MS (Murashige & Skoog, 1962), C17 (Wang & Hu, 1984), or W14 media (Ouyang et al., 1989). Although in the pioneering work of Rines (1983), MS medium without hormones had the highest anther callus initiation rates, the addition of auxin (for example, 2,4-D) was necessary for plant regeneration. The effect of different 2,4-D concentrations on embryo initiation and subsequent plant regeneration on anther culture was reported by Kiviharju et al. (2000). Regenerable types of ELS were produced on nine of the fifteen induction media tested, and four of these promoted plant regeneration. Successful induction of androgenesis on liquid, solid, or double-layer induction medium W14 of F3 generations hexaploid oat hybrids was reported by Ślusarkiewicz-Jarzina and Ponitka (2007). Eight genotypes from fifteen tested produced embryo-like structures, and only two genotypes produced plants. The highest regeneration rate reported so far was described by Kiviharju et al. (2005), who obtained 30 green plants from the Aslak × Lisbeth cross. These experiments clearly show that the response of anthers in culture is determined to a large extent by the genotype of the donor plants, which researchers unanimously emphasize (Kiviharju et al., 2017; Warchoł et al., 2021a). Moreover, experiments conducted on cereals showed that the percentage of anthers producing ELS and the number of haploid plants produced per anther appear to be determined independently by specific genetic locus (Dunwell, 2010).
The present study compares the effects of different genotypes, pre-treatment and post-treatment of the anther, and induction media on androgenic response and plant regeneration from twelve Polish oat (Avena sativa L.) genotypes.
. Materials and methods
Plant growth conditions
The seeds of twelve oat (Avena sativa L.) genotypes (Table 1) were obtained from Strzelce Plant Breeding Ltd. Donor plants were grown from seeds sown individually into pots (⌀ 17 cm) with a soil mixture containing peat substrate, garden soil and sand (3:2:1). Vegetation took place in an air-conditioned greenhouse chamber at a temperature of 21/18 °C (day/night), under natural light. Additionally, for maintaining a 16 hour photoperiod, plants were illuminated (400 µmol m−2 s−1) with high-pressure sodium (HPS) lamps SON-T+ AGRO (Philips, Brussels, Belgium). Plants were fertilized with Hoagland and Arnon (1938) nutrient solution at weekly intervals.
Table 1
Oat (Avena sativa L.) genotypes used for induction of androgenesis.
Panicles pre-treatment
Oat panicles were cut approximately 4 cm above the base of the second leaf, and at the time when it was possible to feel the top of panicles in the leaf sheath. After removing the leaves (except the flag leaf), the panicles were wrapped in plastic bags and aluminum foil and stored in jars with tap water (temperature of 4 °C, on refrigerated counters, under dark conditions). In the first experiment, panicles were cooled for one, two, and three weeks, whereas in the second and third experiments, they were cooled for three weeks. After cooling, panicles were removed from the leaf sheaths, placed in sterile conical flasks, and rinsed with 70% ethanol for 60 s, followed by a 2.0% solution of calcium hypochlorite for 15 min and rinsed 4–5 times with sterile distilled water.
Culture conditions
In the first experiment, temperature post-treatment on androgenesis induction was tested. Anthers were placed on the solid W14 medium (Ouyang et al., 1989) with 5.0 mg L−1 2,4-D and 0.5 mg L−1 benzylaminopurine (BAP), 9% maltose, solidified with 0.3% Phytagel and induced in the dark, at 32 °C or 22 °C for 5 days. Then, anthers from both treatments were divided in half and transferred to a growth chamber with temperatures of 28 °C and 22 °C.
In the second experiment, the effect of media fluidity on androgenesis induction was tested. Anthers were induced in the dark, at 32 °C for five days and then at 22 °C until the end of the experiment, and placed on solid W14 media (with 0.3% Phytagel), semi-liquid W14 media (with 0.15% Phytagel), or liquid W14 media (with 30% Ficoll) (Kiviharju et al., 2005). All media contain 5.0 mg L−1 2,4-D and 0.5 mg L−1 BAP, 9% maltose.
In the third experiment, the effect of media composition on androgenesis induction was tested. Anthers were induced in the dark, at 32 °C for five days and then at 22 °C until the end of the experiment. Anthers were induced on different types of solid media: C17 (Wang & Hu, 1984), W14-1 modified according to Kiviharju et al. (2005) containing 5.0 mg L−1 2,4-D and 0.5 mg L−1 kinetin (KIN), or W14-2 containing 5.0 mg L−1 2,4-D and 0.5 mg L−1 BAP, 50 mg L−1 L-cysteine, 500 mg L−1 myo-inositol, and 20 mg L−1 2-chloroethylphosphonic acid (ethephon). All media containing 9% maltose were solidified with 0.3% Phytagel and autoclaved for 20 min at 120 °C. Growth regulators and vitamins were sterilized by filtration and added to the medium chilled after autoclaving.
In all experiments, anthers were placed in Petri dishes (⌀ 6 cm) containing approximately 10 ml of induction media. Anthers were isolated from the middle part of tillers, omitting their top and bottom. Additionally, to induce embryogenesis, 5–7 immature ovaries were added to each Petri plate. Induced calluses and ELS were separated from anthers and transferred to W14 medium (Ouyang et al., 1989) with 2.0 mg L−1 2,4-D and 0.5 mg L−1 KIN, 9% maltose, and 0.6% agar, and regenerated plants to solid ½ MS medium (Murashige & Skoog, 1962) with 3% sucrose and without growth regulators. Regeneration was carried out at light intensity of 80–100 µmol⋅m−2⋅s−1, a 16 hour photoperiod, and a temperature of 22 °C. After about four weeks, the rooted plants were planted into containers with perlite moistened with liquid Hoagland and Arnon (1938) medium for about three weeks.
Chromosome doubling and plants acclimation
Chromosome doubling was carried out by placing the well-rooted plants in a solution composed of 0.1% colchicine, 4% dimethyl sulfoxide, 0.3% Tween, and 25 mg L−1 gibberellic acid for 7.5 hours at 25 °C and a light intensity 80–100 µmol⋅m−2⋅s−1. After that, the plants were rinsed continuously for 48 hours in a stream of running water. Finally, plants were grown in 3 L pots filled with a mixture of peat, garden soil, and sand (3:2:1) in a greenhouse at 21/18 °C and at a 16 hour photoperiod. Plants were illuminated (400 µmol m−2 s−1) with high-pressure sodium (HPS) lamps SON-T+ AGRO (Philips, Brussels, Belgium).
Assessment of anthers and microspores viability
Upon isolation from panicles, anthers were green. After four weeks of culture, their viability was assessed on a 4-point scale, where 1 represents green anthers, 2 – yellow anthers, 3 – light brown anthers, and 4 – brown anthers. To determine the viability and developmental stages of microspores at the time of isolation and subsequent phases of microspore development in in vitro cultures, the cytological observations were performed using a light microscope (SMZ 1500, Nikon, Tokyo, Japan). Anthers were isolated from the middle part of a freshly cut panicle and crushed on a glass slide in a drop of water.
Plants ploidy analysis
Oat plant leaf samples were prepared for flow cytometric analysis using the method described by Galbraith et al. (1983). Propidium iodide (50 µg mL−1) and ribonuclease A (50 µg mL−1) were added to the nuclei isolation buffer in order to stain DNA. A high-grade solid-state laser emitting green light at 532 nm was fitted with a CyFlow SL Green flow cytometer (Partec GmbH, Münster, Germany) to quantify the fluorescence of nuclei in each sample. The program FloMax (Partec GmbH, Münster, Germany) was used to analyze the histograms.
Statistical analysis of results
A completely randomized design was applied in the experiments with 12 genotypes. Each experiment had 15 replications (1 oat plant per 1 pot) for each oat genotype. The statistical significance of differences between the tested experimental objects was assessed based on analysis of variance in a completely random design, using Duncan’s test at p < 0.05. Statistical analysis was performed using the STATISTICA 10.0 package (Stat-Soft, Inc., USA) for Windows.
. Results
The influence of the cooling period at 4 °C of oat panicles on the viability of anthers
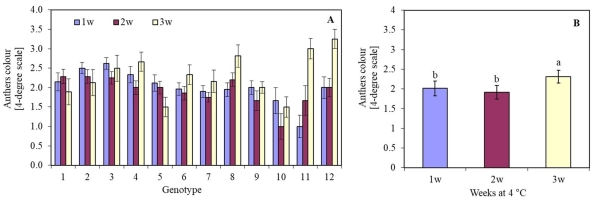
The length of the cooling period (1, 2, or 3 weeks) of oat panicles at 4 °C significantly influenced the viability of anthers in most genotypes. The darkening and death of anther tissues depended on the oat genotype. Genotype 10 (Sławko × Kasztan) was characterized by the highest viability of anthers tissue. Anthers of genotypes 4 (Flämingsprofi × STH 238), 8 (STH 65488 × Chimene), 11 (Chwat × Szakal), and 12 (Flämingsprofi × Matilda) cooled for three weeks, darkened, and died much faster compared to the remaining genotypes tested (Figure 1A). By averaging the results of anther viability of all genotypes, it was observed that after one or two weeks of cooling panicles, anthers remained viable longer (Figure 1B) than after three weeks of cooling.
Figure 1
The colour of oat (Avena sativa L.) anthers, expressed in a 4-degree scale (1 – green anthers, 2 – yellow anthers, 3 – light brown anthers, and 4 – brown anthers), depending on (A) the genotype and panicles pre-treatment period (1, 2, or 3 weeks) in 4 °C; (B) mean values for all genotypes pre-treated in 4 °C for 1, 2 and 3 weeks. Bars represent mean values ± SE. Different letters denoted significant differences between treatments at p < 0.05. The tested genotypes are listed in Table 3.
The influence of temperature on the viability of oat anthers and the induction of androgenesis
It was shown that most of the microspores on the day of isolation were in the late-uninucleate stage, with the cell nucleus located at the pole opposite to the operculum (Figure 2A). The cell nucleus was usually located adjacent to the wall in a narrow band of cytoplasm. The lenticular generative cell located adjacent to the cell wall was also visible. A large, single vacuole occupied a significant volume of the cell.
Figure 2
Stages of androgenesis in oat (Avena sativa L.) anther culture. (A) microspores at the uninucleated (UN) and binucleated (BN) stages; gc - generative cell, n - nucleus, op – operculum, v – vacuole; (B) anthers culture on W14-1 medium; (C) star-like microspores indicated by blue arrows and accumulating starch grains indicated by black arrows; (D) anthers with developed embryo-like structure indicated by arrow; (E) calluses separated from anthers on W14 medium; (F) regenerated plant on ½ MS medium; (G) acclimatization of regenerated plant in perlite; (H) acclimatized plants transferred to soil; (I) DH plant of genotype 10 (Sławko × Kasztan).

Conducting anther culture for five days at 32 °C resulted in a decrease in viability compared to anthers that were not subjected to this temperature (Figure 2B). The anther viability was at the same level regardless of the temperature only in the case of two genotypes: 9 (Sławko × Matilda) and 10 (Sławko × Kasztan). Treatment with a temperature of 32 °C on anthers of genotypes 1 (Belinda × STH 5244), 2 (Flämingsprofi × STH 5244), 4 (Flämingsprofi × STH 238), 5 (STH 132 × Matilda), 7 (STH 238 × Matilda), and 12 (Flämingsprofi × Matilda) significantly worsened their colour. Only in genotype 3 (Flämingsprofi × STH 132) applied temperature of 32 °C resulted in the increase of anther viability (Figure 3A). By averaging the results of anther viability for all genotypes tested, it was observed that the temperature of 32 °C caused a significant decrease in their viability (Figure 3B).
Figure 3
The colour of oat (Avena sativa L.) anthers, expressed in a 4-degree scale (1 – green anthers, 2 – yellow anthers, 3 – light brown anthers, and 4 – brown anthers), depending on (A) the genotype and temperature of anthers pre-treatment in 22 °C and 32 °C for 5 days; (B) mean values for all genotypes in 22 °C and 32 °C for 5 days; (C) the genotype and temperature of anthers induction in 22 °C and 28 °C; (D) mean values for all genotypes induced in 22 °C and 28 °C. Bars represent mean values ± SE. Different letters denoted significant differences between treatments at p < 0.05.
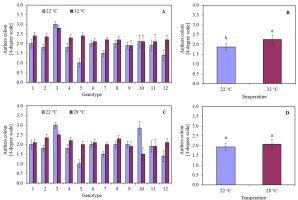
It was observed that the temperature of 28 °C in most of the investigated genotypes had a worse effect than the temperature of 22 °C on the callus colour. This was particularly visible in the case of genotypes 2 (Flämingsprofi × STH 5244), 4 (Flämingsprofi × STH 238), 5 (STH 132 × Matilda), 7 (STH 238 × Matilda), and 12 (Flämingsprofi × Matilda) (Figure 3C). However, in genotypes: 3 (Flämingsprofi × STH 132) and 10 (Sławko × Kasztan), greater anther viability was observed during induction at 28 °C. On average, it was observed that treating anthers with a temperature of 28 °C did not significantly affect their viability compared to 22 °C. The degree of anther viability remains at a similar level (Figure 3D). Despite the developmental phase of microspores that is appropriate for the induction of androgenesis, most of them did not show induction of sporophyte development. Even initially enlarged cells accumulated starch grains during the first few days and gradually died (Figure 2C).
Starting from the third week of in vitro culture, single, multi-cellular cellular structures similar to callus tissue were observed in genotypes 1 (Belinda × STH 5244), 4 (Flämingsprofi × STH 238), 7 (STH 238 × Matilda), 8 (STH 65488 × Chimene), 10 (Sławko × Kasztan), 11 (Chwat × Szakal), and 12 (Flämingsprofi × Matilda). However, after approximately five weeks of culture, most of the calluses died, and no androgenic structures capable of regeneration were obtained. These structures were observed only in the case when, before isolating the anthers, the panicles were cooled at 4 °C for two weeks, and the anthers were induced at the temperature of 32 °C for five days, followed by a temperature of 28 °C.
The influence of medium fluidity on the induction of oat androgenesis
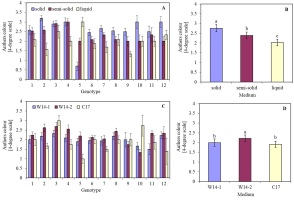
The effect of the medium on anther viability depended on the genotype. Although the highest anther viability on each of the tested media was detected in genotype 9 (Sławko × Matilda), it could be observed that the viability of four other genotypes: 1 (Belinda × STH 5244), 6 (STH 238 × NK 03011), 7 (STH 238 × Matilda), and 8 (STH 65488 × Chimene), did not differ much from its values (Figure 4A). Moreover, the fastest anthers browning of all tested genotypes was found on a solid medium, with viability assessed at the level of 2.5–3 relative units on a 4-point scale. Anthers incubated in a liquid medium were characterized by the highest viability.
Figure 4
The colour of oat (Avena sativa L.) anthers, expressed in a 4-degree scale (1 – green anthers, 2 – yellow anthers, 3 – light brown anthers, and 4 – brown anthers), depending on (A) the genotype and media solidification; (B) mean values for all genotypes on a solid, semi-solid, and in a liquid medium; (C) the genotype, and kind of medium; (D) mean values for all genotypes on W14-1, W14-2, and C17 medium. Bars represent mean values ± SE. Different letters denoted significant differences between treatments at p < 0.05.
After approximately five weeks of culture, five genotypes, including 1 (Belinda × STH 5244), 4 (Flämingsprofi × STH 238), 7 (STH 238 × Matilda), 8 (STH 65488 × Chimene), and 11 (Chwat × Szakal), formed callus structures even on completely browned anthers (Table 2, Figure 2D). Only in genotype 4 (Flämingsprofi × STH 238) single, called star-like microspores, were observed with a cell nucleus located adjacent or centrally and with distinct cytoplasmic bands crossing the vacuole and connecting the cytoplasm around the nucleus with a narrow band of cytoplasm at the cell periphery (Figure 2C). It was observed that the induction of callus and ELS was not dependent on the degree of anther darkening. Obtained callus structures were then separated from the anther tissues and transferred to the regeneration medium (Figure 2E). However, after subsequent weeks of culture, most of the callus structures died. In total, over six thousand isolated anthers, six calluses, and one plant of genotype 4 (Flämingsprofi × STH 238) were obtained (Table 2).
Table 2
Induction of oat (Avena sativa L.) androgenesis depending on the medium solidification (number of panicles, anthers, calluses, embryo-like structures, haploids, and doubled haploid plants).
| Genotype | Panicles | Solid medium | Semi-solid medium | Liquid medium | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anthers | Callus (ELS*) | Haploids (DH**) | Anthers | Callus (ELS) | Haploids (DH) | Anthers | Callus (ELS) | Haploids (DH) | ||
| 1 | 41 | 795 | – | – | 1027 | – | – | 1027 | 2(–) | – |
| 2 | 50 | 1319 | – | – | 857 | – | – | 1165 | – | – |
| 3 | 45 | 731 | – | – | 889 | – | – | 1348 | – | – |
| 4 | 6 | 27 | – | – | 128 | 1(1) | 1 | 174 | – | – |
| 5 | 10 | 401 | – | – | 45 | – | – | 129 | – | – |
| 6 | 41 | 866 | – | – | 625 | – | – | 1009 | – | – |
| 7 | 37 | 709 | – | – | 569 | 1(–) | – | 729 | – | – |
| 8 | 37 | 813 | 1(–) | – | 771 | – | – | 681 | – | – |
| 9 | 6 | 81 | – | – | 84 | – | – | 162 | – | – |
| 10 | 10 | 82 | – | – | 242 | – | – | 339 | – | – |
| 11 | 10 | 164 | – | – | 218 | 1(–) | – | 331 | – | – |
| 12 | 6 | 75 | – | – | 96 | – | – | 195 | – | – |
| Σ | 299 | 6063 | 1(–) | – | 5551 | 3(1) | 1 | 7289 | 2(–) | – |
The influence of the type of medium on the induction of oat androgenesis
The effect of the type of medium on anther viability also depended on the genotype. The highest anther viability on all tested media was observed in genotypes 5 (STH 132 × Matilda), 6 (STH 238 × NK 03011), and 7 (STH 238 × Matilda). Their anthers remained green for the longest period. In turn, the faster darkening on all media tested was noticed for anthers of genotype 3 (Flämingsprofi × STH 132). It was also observed that on the W14-2 medium, anthers darkened faster than on the W14-1 and C17 media.
Induction of callus and ELS was observed in seven genotypes out of the twelve examined (Table 3). Although the largest number of callus structures were obtained from genotype 8 (STH 65488 × Chimene), the regeneration of DH plants was not recorded. Regeneration of haploid plants from calluses (Figure 2F–G) was possible for genotypes 4 (Flämingsprofi × STH 238), 7 (STH 238 × Matilda), 8 (STH 65488 × Chimene), and 10 (Sławko × Kasztan). Still, among the seven haploid plants (Figure 2H), only two DH plants (Figure 2I) of genotypes 4 (Flämingsprofi × STH 238), and 10 (Sławko × Kasztan) were obtained. These two DH plants were regenerated on W14-1 and C17 medium.
Table 3
Induction of oat (Avena sativa L.) androgenesis depending on the kind of medium (number of panicles, anthers, calluses, embryo-like structures, haploids, and doubled haploid plants).
| Genotype | Panicles | W14-1 medium | W14-2 medium | C17 medium | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anthers | Callus (ELS*) | Haploids (DH**) | Anthers | Callus (ELS) | Haploids (DH) | Anthers | Callus (ELS) | Haploids (DH) | ||
| 1 | 39 | 1226 | – | – | 1313 | 1(–) | – | 260 | – | – |
| 2 | 46 | 1865 | – | – | 1788 | – | – | 204 | – | – |
| 3 | 52 | 1921 | 2(–) | – | 1521 | – | – | 78 | – | – |
| 4 | 21 | 599 | 3(3) | 3(1) | 489 | – | – | 164 | – | – |
| 5 | 34 | 1012 | – | – | 913 | – | – | 72 | – | – |
| 6 | 49 | 1323 | – | – | 1594 | – | – | 54 | – | – |
| 7 | 66 | 2049 | 1(1) | 1(–) | 1864 | 1(1) | 1(–) | 196 | – | – |
| 8 | 52 | 1693 | 6(–) | – | 1721 | 1(1) | 1(–) | 22 | – | – |
| 9 | 8 | 243 | – | – | 228 | – | – | 935 | – | – |
| 10 | 6 | 213 | – | – | 188 | – | – | 1126 | 2(1) | 1(1) |
| 11 | 10 | 473 | – | – | 330 | – | – | 554 | 1(–) | – |
| 12 | 21 | 725 | – | – | 596 | – | – | 453 | – | – |
| Σ | 404 | 13342 | 12(4) | 4(1) | 12545 | 3(2) | 2(–) | 4118 | 3(1) | 1(1) |
The ploidy of the regenerated plants was compared to control diploid oat plants (Figure 5A), both before and after, the plants were treated with colchicine (Figure 1B–C). The studied plants had doubled chromosomes due to the colchicine treatment, according to cytometric examination.
Figure 5
Flow cytometry histograms showing: (A) control 2n; (B) haploid 1n; (C) doubled haploid 2n of oat plants.

In turn, among the three types of media tested, the most efficient was W14-1, on which anthers produced 12 calluses and 4 ELS, while on W14-2 and C17 media, three calluses and two or 1 ELS were produced, respectively. To summarize, with over 13 thousand isolated anthers, only 18 calluses, seven ELS, seven haploid plants, and two DH were obtained.
. Discussion
The method of androgenesis is an increasingly utilized technique in cereals to produce homozygous plants, whereby the in vitro generation of androgenic embryos proves to be a more efficient procedure than gynogenesis-based techniques (Seguí-Simarro et al., 2021). Obtaining oat (Avena sativa L.) doubled haploids (DH) from unfertilized male germ cells is considerably more challenging compared to other cereals. Although there are several reports on successful oat regeneration via androgenesis (Kiviharju et al., 2005; Rines et al., 1997; Warchoł et al., 2019), an efficient method for obtaining haploids of this species in both isolated microspore cultures and anther cultures has not yet been developed. Previous research also indicates that microspores are most sensitive to androgenesis induction during the late uninucleate or early binucleate stage following division. Abiotic stress appears to influence the differentiation of microspores in the period leading up to the start of culture.
Pre-treatment of oat panicles
De Cesaro et al. (2009) have identified high- or low-temperature, sugar- and nitrogen-free media, as well as treatment with mannitol, heavy metal ions, or colchicine, among the most applied stress factors. In a pioneering study, Rines (1983) has demonstrated that low temperature does not affect the efficiency of androgenesis in oat. Similar observations were described by Kiviharju and Pehu (1998), where a 7-day cold treatment (4 °C) of oat panicles resulted in callus production but did not affect the formation of embryogenic structures or the regeneration of haploid plants. Warchoł et al. (2019) reported different relationships since the low-temperature stress applied to plants before isolating anthers can stimulate the microspore redirection from their gametophytic to the sporophytic developmental pathway. The results from the study presented here confirmed these findings, demonstrating that a temperature of 4 °C applied for seven and 14 days not only induced callus production but also affected the formation and regeneration of ELS. In addition, the duration of the cold treatment period also determines anther darkening and microspore viability. Research conducted by Kiviharju et al. (1998) showed that cold treatment of the panicles for seven and 14 days at 4 °C was the most optimal for induction of anther cultures, characterized by the highest degree of viability. However, extending this process to 21 days resulted in the browning of the anthers and a decrease in microspore viability. Since a similar relationship was observed in our study, in the subsequent experiment, we shortened the cold treatment period of the panicles to two weeks.
According to Immonen and Anttila (1999), prolonged (from 21 to 28 days) cold storage of cereal panicles at 4 °C before placing the anthers on the medium is a factor that stimulates the reprogramming of microspores, especially in androgenesis-resistant genotypes. Although no differences in response to the cold treatment period were observed between genotypes in our study, a 3-week treatment of panicles with a temperature of 4 °C proved to be unfavorable for all tested genotypes. Haploid oat plants were obtained only when panicles were cold treated for 2 weeks before anther isolation. Similar results were obtained by Warchoł et al. (2019), who obtained DH lines only when the cold treatment of panicles at 4 °C was shortened from three to two weeks.
Szarejko (1997) indicated a negative impact of low temperatures on barley. The duration of panicle cold treatment did not significantly stimulate the process of androgenesis or increase the frequency of regenerated green plants. On the contrary, prolonged exposure to low temperatures (28–30 days) drastically reduced the effectiveness of the applied method. A similar relationship was observed in our study, where after 3 weeks of low-temperature treatment of oat panicles, anthers turned brown, and microspore viability decreased, whereas low-temperature treatment for seven to 14 days could stimulate the transition of microspores towards androgenesis. However, it was observed that for several genotypes, extending the cold treatment period resulted in increased anther viability. On this basis, it can be inferred that there is an interaction between the cold treatment and the genotype tested.
Anther culture conditions
In addition to the preliminary treatment of panicles with low or high temperatures for the induction of androgenic embryos in cereals, similar procedures can also be applied to isolated anthers. Rines (1983) exposed isolated oat anthers to high temperatures (35 °C) for 12 hours, followed by incubation at 32 °C for five days. It appeared that high-temperature post-treatment negatively affected the plant regeneration rate compared to cultures in which 32 °C incubation was omitted. However, the temperature shock significantly reduced the number of albino plants. The influence of high temperature on androgenesis induction in oat was described by Kiviharju and Pehu (1998), who demonstrated that treating isolated anthers with a temperature of 32 °C for five days was optimal for Avena sativa L. and Avena sterilis L., resulting in the induction of the highest number of haploid embryos. When the treatment time was extended to seven days, the number of Avena sativa L. embryos was two times lower, and higher callus production was observed in the culture. Despite the fact that the application of heat shock did not lead to the production of haploid plants, the authors emphasized that high-temperature treatment could be a breakthrough in successful oat anther cultures. This was consistent with a study conducted by Warchoł et al. (2021b), where haploid plants of oat cultivar ‘Chwat’ were obtained by cold treating the panicles for 14 days at 4 °C, followed by 24 hours at 32 °C. In the study of Ferrie et al. (2014), embryos and ELS were produced within each temperature range tested, with treatment at 28 °C generating noticeably more ELS. Based on a preliminary study in which a range of temperatures were tested, oat microspores showed swelling but no further development in response to heat shock at 32 or 35 °C. In contrast, microspores cultured at 28 °C did produce multi-cellular structures. Further studies conducted by Kiviharju et al. (2005) confirmed that incubating anthers at temperatures higher than 30 °C increased the number of ELS while lowering the temperature to 28 °C was more favourable for ELS regeneration. In the present study, the relationships described above were not observed. However, it was found that the temperature of 32 °C applied during the incubation had a favourable effect on the colour of the anthers, yet the chances of androgenesis initiation did not diminish. Although fewer callus structures were obtained using the aforementioned temperature, it proved to be effective in producing haploid plants and, ultimately, DH lines. Kiviharju et al. (2000) have suggested that to enhance the efficiency of androgenesis, the procedure for pre-treating anthers must be genotype-specific. Additionally, authors observed that a high 2,4-D concentration in the medium was a factor increasing the induction of haploid embryos in all tested genotypes, regardless of whether the anther culture was subjected to high temperature or not.
Induction media
According to the literature, apart from the temperature of anther pre- and post-treatment, the composition of the inductive medium is one of the most frequently modified factors influencing the efficiency of oat androgenesis. The media’s mineral salt levels, carbon and nitrogen sources, and concentrations, liquid versus solidified media, and different growth regulators all play crucial roles in determining the number of ELS, haploid embryos, and regenerated plants. The first successful experiments on oat androgenesis were conducted by Rines (1983). Among the various media tested, hormone-free MS medium supplemented with 10% sucrose induced the highest rate of anther callus initiation. However, seedlings were only generated from anthers that were grown on a modified potato extract medium containing 0.5 mg L−1 kinetin (KIN) and 2.0 mg L−1 2,4-D. This experiment demonstrated a dramatically low rate of regeneration of oat haploid plants obtained through androgenesis. Subsequently, Sun et al. (1991) obtained twelve green regenerants of Avena sativa L. and one albino regenerant of naked oat (Avena nuda L.). Anthers were isolated onto MS medium supplemented with 1.0 mg L−1 2,4-D, while for ELS regeneration, MS medium enriched with 0.1 mg L−1 NAA and 0.5 mg L−1 BAP was used. The final outcome of the conducted experiments was the acquisition of two haploid and one euploid plant.
In the following years, the method of obtaining embryogenic structures was refined through modifications of the composition of induction media and variations in the physical conditions during pollen culture (Kiviharju et al., 1997, 1998; Ponitka & Ślusarkiewicz-Jarzina, 2009; Rines et al., 1997). A significantly increased regeneration rate from oat anther culture was reported by Kiviharju et al. (2005). In this study, a W14 induction medium containing 2,4-D, BAP, ethephon, cysteine, and myo-inositol resulted in significantly higher rates of green plant regeneration for the tested cultivars ‘Aslak’ and ‘Lisbeth’ compared to the media containing only 2,4-D and KIN. This indicated that the combined application of 2,4-D and BAP improved the quality of ELS. Contradictory results were obtained in the present study, where W14-1 medium containing 2,4-D and KIN proved to be superior compared to W14-2 containing 2,4-D and BAP. Anthers cultured on the W14-1 medium exhibited higher viability compared to those cultured on the W14-2 medium, resulting in a higher number of ELS and regenerated plants. These findings were confirmed by the research of Warchoł et al. (2019), in which the addition of 2.0 mg L−1 of 2,4-D and 0.5 mg L−1 of KIN to the W14 medium resulted in the highest number of ELS and haploid plants.
The present study also assessed the effect of medium solidification degree on anther colour, ELS induction, and plant regeneration. The results clearly indicate that anthers remained green for the longest time in the liquid medium, while on the solid medium, most of them turned brown. However, the browning of the anthers connected with the oxidation of phenolics during culture did not correlate with the viability of microspores and their ability to produce ELS. Different findings were described by Ślusarkiewicz-Jarzina and Ponitka (2007), who obtained the highest number of haploid plants on a solid agar medium. Authors also demonstrated that anthers cultured on a solid medium produced more ELS than those cultured in a liquid medium. According to Szarejko (1997), submersion of anthers in the medium could be one of the reasons for the loss of microspore ability to form ELS in liquid medium, creating anaerobic conditions unfavourable for regeneration processes. In the current experiment, adding 30% Ficoll to a liquid medium increased their density, causing anthers to float on the surface.
. Conclusions
The low efficiency of androgenesis in oat anther culture means that this method has no practical application in breeding and requires further research to improve it. In our investigation, heat post-treatment, along with a medium supplemented with 2,4-D, KIN, and maltose as a carbohydrate source, enhanced ELS induction, suggesting the potential influence of multiple induction factors on oat androgenesis. As previously hypothesized by Kiviharju et al. (2005), not only the above-mentioned factors significantly limit the androgenic response in oat, but also the genotype and irregular maturation of anthers. In the presented work, from over 13 thousand isolated anthers, only 18 calluses, seven ELS, seven haploid plants, and two DH were obtained. These results show that it is possible to induce ELS, but further research that will lead to an increase in androgenesis efficiency and successful regeneration of doubled haploids is necessary.


