. Introduction
Lilium candidum L. (Madonna lily) is a bulbous geophyte native to the Mediterranean area. It has been eagerly cultivated due to its properties, so the exact place of origin is difficult to determine (Özen et al., 2012; Zaccai et al., 2009). Its wide use resulted from its medicinal and ornamental attributes and cultural significance, mainly as a symbol of the Marian cult (Pałka et al., 2023a). The positive, anti-inflammatory, and anticancer properties of Madonna lily have been confirmed by modern scientific research (Patocka et al., 2019). Moreover, Madonna lily is, among others, a source of phenolic acids with documented antimicrobial, antioxidant, and skin healing effects (Pałka et al., 2023b).
Regeneration of wild populations and generative reproduction in this species are difficult due to self-sterility, and conventional vegetative reproduction shows low efficiency (Mynett, 1992; Patil et al., 2021). There have also been relatively few works on in vitro cultures of Madonna lily (Altan & Bürün, 2017; Altan et al., 2010; Burun & Sahin, 2013; Khawar et al., 2005; Pałka et al., 2023a, 2023b; Patil et al., 2021; Saadon & Zaccai, 2013; Sevimay et al., 2005; Tokgöz & Altan, 2020). Only one work involved liquid medium cultures (Daneshvar Royandazagh, 2019). So far, no research has been conducted regarding the use of bioreactors for the propagation and cultivation of L. candidum.
Liquid and bioreactor cultures were used for other species of the genus Lilium. Liquid media enable rapid multiplication of biological material, and the use of bioreactors allows for automation and reduction of production costs (Bakhshaie et al., 2016; Lian et al., 2014). Plant growth regulators are more effective in a liquid medium due to a lack of binding by gelling agents (Barberini et al., 2011). Direct contact of explants with the medium also contributes to the increased availability of other medium components. A disadvantage of systems based on liquid media is often limited aeration and the resulting possibility of hyperhydricity (Mirzabe et al., 2022; Ziv, 2005).
RITA®; is a bioreactor with a temporary immersion system (TIS), which consists of a single vessel divided into two parts: the upper one being a culture chamber, where the cultivated plants are periodically immersed in the nutrient medium, and the lower one serving as the medium storage tank, into which the medium flows in the resting phase of the bioreactor when the flow of airlifting the medium is stopped (Mirzabe et al., 2022; Robert et al., 2006). The immersion time is usually short and lasts a few minutes. The selection of immersion time and the medium components allows for obtaining high multiplication rates and avoiding undesirable outcomes, such as hyperhydricity (de Carlo et al., 2021) and asphyxia (Etienne & Berthouly, 2002). One of the most critical factors for biomass accumulation is immersion frequency (Pérez-Alonso et al., 2009). In bioreactor cultures of Hippeastrum × chmielii Chm. (Ilczuk et al., 2005) and Leucojum aestivum L. (Ptak, 2014), higher propagation rates were obtained in a bioreactor than in traditional culture on semi-solid or solid media. The aim of the study was to determine the impact of pretreatment in the RITA bioreactor (duration and frequency of the liquid medium immersion and number of weeks of pretreatment) on the in vitro organogenesis and biochemistry of L. candidum bulbscales.
. Material and methods
Plant material
Single bulbscales of Lilium candidum L. weighing 60 ± 10 mg were used as explants for the experiment. They were isolated from 11–12 scaled adventitious bulbs cooled in in vitro culture at 4 °C for 12 months (Pałka et al., 2023a).
Experimental set
The experiment examined the influence of bulbscale pretreatment with a liquid medium in the RITA bioreactor (pretreatment) on bulbscale regeneration on a solid medium (regeneration) (Figure 1).
Figure 1
Scheme of an experiment consisting of two parts: pretreatment in a RITA bioreactor and culture on a solid medium.

During pretreatment, four combinations of frequency and immersion time were tested: three times a day for 1 minute (RITA 3 × 1), 3 times a day for 5 minutes (RITA 3 × 5); once a day for 15 minutes (RITA 1 × 15), and 3 times a day for 15 minutes (RITA 3 × 15). Periods of immersion occurred at equal time intervals in the RITA bioreactor. The pretreatment lasted 1, 2, 3, 4, 5, or 6 weeks. As many as 30 bulbscales were placed in each vessel of the RITA®; bioreactor (Vitropic, France) containing 200 mL of the medium. Each combination consisted of 6 RITA vessels, each of which was a separate repetition. Each combination contained 180 bulbscales.
After pretreatment in the bioreactor, the bulbscales (explants) were transferred to 100 mL glass flasks sealed with aluminum foil, containing 30 mL of a solid medium (regeneration) for six weeks. Five explants were placed in each flask. For each combination, three flasks were prepared, each being a separate repetition. Combination without treatment in RITA was a control.
The experiment used medium (pretreatment and regeneration) containing macroelements, microelements, and vitamins according to Murashige and Skoog (MSS) (1962), growth regulators: 5 µM BAP and 0.5 µM NAA (Duchefa Biochemie, Netherlands), and 3% sucrose. The solid medium was solidified with 0.5% BioAgar (BIOCORP, Poland). The medium pH was set at 5.8. The cultures were carried out in a phytotron at a temperature of 19/17 °C (day/night), relative humidity 80%, and 16 h photoperiod (16 h day/8 h night), under a fluorescent lamp (OSRAM L 36W/77 FLUORA), with a PPFD of 30 µmol m2 s−1.
Data collection
After 1, 2, 3, 4, 5, and 6 weeks of pretreatment and at the end of the 6-week regeneration, we determined the following parameters: biomass weight, biomass growth index, single bulblet weight, bulblet dry weight, number of bulblets produced on the explants, soluble sugar content in the bulblets, and soluble sugar content in the liquid medium. The biomass growth index was determined using the following formula: (final fresh weight – initial fresh weight)/initial fresh weight.
To determine the content of soluble sugars and dry matter in the bulblets, three 1 g samples were prepared from each combination. Three 1 mL samples of the liquid medium were also taken to analyze the content of soluble sugars. All samples were frozen at −80 °C. The frozen bulblets were freeze-dried (Freezone 4.5 freeze dryer, Labconco, USA). The percentage of dry matter in the bulblets was calculated based on the weight of the freeze-dried material.
Content of soluble sugars
To analyze soluble sugar content, the freeze-dried bulblets were homogenized in a QIAGEN TissueLyser II homogenizer (QIAGEN, Germany). Then, three 3 mg samples were macerated in 1 mL of distilled water for 12 hours in a refrigerator. After centrifugation (Eppendorf Centrifuge 5415 R, Bioridge Centrifuge, China), 0.2 mL samples were collected for an analysis based on the anthrone method (Dische, 1962). Absorbance values were measured at 620 nm in an Amersham Biosciences Ultrospec2100 pro spectrophotometer (Amersham Biosciences, UK). Based on glucose solutions of known concentrations, a calibration curve was created, and the content of soluble sugars in the tested material was calculated. The same method was used to analyze the content of soluble sugars in the medium, samples of which had previously been thawed at room temperature. By dissolving 5 µL of the medium in 2 mL of distilled water, a working dilution was obtained, from which 0.2 mL samples were taken for analysis.
Statistical analysis
All the study findings were analyzed statistically using the Statistica 13.3 software (StatSoft, TIBCO Software Inc., Palo Alto, CA, USA). The experiment was completely randomized.
To assess the character and strength of relations between the parameters, the Pearson correlation coefficient (r) (Khamis, 2008; Kornbrot, 2014; LeBlanc & Cox, 2017) was applied. Later on, the analysis of variance (ANOVA) was used, followed by the post hoc multiple range Duncan test. Significantly different means were separated at p ≤ 0.05.
. Results
The effects of the pretreatment time of L. candidum bulbscales with the liquid medium in the RITA®; bioreactor on the formation of bulblets during regeneration on the solid medium are presented in Figure 2. During both experiment stages (pretreatment and regeneration), adventitious organogenesis of L. candidum bulbscales was observed (Table 1). The bulblets began to form on the explants cultivated in the bioreactor for a minimum of two weeks, in the third week of culture, regardless of the immersion frequency (Table 1, Figure 2).
Figure 2
Lilium candidum L. bulbscales after the 1st (A), 3th (C), 5th (E) and 6th (G) week in RITA 3 × 15 pretreatment (immersion 3 times a day for 15 minutes) and then after 6 weeks of culture on solid medium (B, D, F and H). Bar = 1 cm.

Table 1
Effects of pretreatment time and immersion frequency on the number of Lilium candidum bulblets on explants. Statistical effect for the source of variation (weeks, frequency, weeks × frequency) for p ≤ 0.001.
| Stage of experiment | Weeks in RITA | Immersion frequency | |||
|---|---|---|---|---|---|
| 3 × 1a | 3 × 5 | 1 × 15 | 3 × 15 | ||
| Pretreatment (liquid medium) | 1 | 0.00ab | 0.00a | 0.00a | 0.00a |
| 2 | 0.00a | 0.00a | 0.00a | 0.00a | |
| 3 | 0.30 ± 0.05b | 0.62 ± 0.08d | 0.74 ± 0.03e | 0.35 ± 0.04b | |
| 4 | 0.54 ± 0.04c | 0.83 ± 0.03f | 1.20 ± 0.03h | 0.77 ± 0.10ef | |
| 5 | 0.73 ± 0.03e | 0.91 ± 0.09g | 1.48 ± 0.08i | 1.83 ± 0.07k | |
| 6 | 0.83 ± 0.00f | 0.97 ± 0.00g | 1.70 ± 0.00j | 2.07 ± 0.00l | |
| Regeneration (6 weeks on solid medium) | 0 (control) | 2.17 ± 0.14c–e | |||
| 1 | 1.29 ± 0.04a | 1.38 ± 0.13a | 1.47 ± 0.19a | 2.33 ± 0.14e | |
| 2 | 1.42 ± 0.16a | 1.47 ± 0.13a | 1.88 ± 0.13bc | 3.23 ± 0.03g | |
| 3 | 1.81 ± 0.17b | 2.00 ± 0.0b–d | 1.88 ± 0.13bc | 3.55 ± 0.30h | |
| 4 | 1.89 ± 0.19bc | 2.08 ± 0.14b–e | 1.92 ± 0.14b–d | 3.79 ± 0.19hi | |
| 5 | 2.11 ± 0.19b–e | 2.36 ± 0.13e | 2.17 ± 0.14c–e | 3.98 ± 0.23i | |
| 6 | 2.22 ± 0.20de | 2.83 ± 0.17f | 2.75 ± 0.25f | 4.02 ± 0.23i | |
Biomass growth index
In the first four weeks of the pretreatment, the biomass growth index was uniform in all tested combinations (Figure 3). Significant weight gains were observed in the 5th and 6th weeks for the cultures immersed in the medium three times a day (for 1, 5, and 15 minutes). The highest biomass growth index was achieved in the cultures immersed three times a day for 5 minutes, while the smallest biomass growth for this time interval was recorded in the cultures immersed once a day (RITA 1 × 15). It turned out that breaking the 15-minute immersion cycle into three 5-minute cycles spaced evenly throughout the day (3 × 5 instead of 1 × 15) more than doubled the biomass growth index from 1.26 to 2.95 (Figure 3A). A longer rest phase (time between immersions) limits biomass growth. In the cultures immersed once a day, those grown on the solid medium, and those immersed three times a day for a very short time of 1 minute, the biomass growth index increase was the lowest and ranged from 1.26 for the frequency of 1 × 15 minutes a day to 1.69 in the case of the cultures immersed 3 × 1 minute per day (Figure 3A–B).
Figure 3
Biomass growth index of Lilium candidum in temporary immersion bioreactor system RITA pretreatment (A) and on solid medium (B). Data are presented as means ± standard deviations. Different letters indicate significant differences between values according to Duncan’s multiple range test at p ≤ 0.05.

In the bulbscale cultures treated with the liquid medium at various frequencies for one to six weeks (pretreatment), further weight gain was observed during regeneration after transfer to the solid medium of the same composition (Figure 3B). After six weeks of regeneration on the solid medium, the highest biomass growth index (2.77) was observed in the cultures immersed in the medium for six weeks at a frequency of 3 × 5 minutes a day. High, but slightly lower values (2.59 and 2.62, respectively) were obtained for the cultures immersed three times a day for 15 minutes for five and six weeks of the pretreatment. The longer the contact time with the medium during pretreatment, the more intense was the growth during regeneration. The cultures immersed three times a day for 15 minutes, transferred after two weeks of pretreatment from the bioreactor to the solid medium, reached a higher biomass growth index than the control scales (not pretreated with the liquid medium). The frequencies resulting in the lowest biomass growth index (lower or comparable to the control) were three times a day for 1 minute and one time a day for 15 minutes, regardless of the cultivation time in the RITA bioreactor (Figure 3B).
Number of bulblets and single bulblet weight
During pretreatment, the highest number of bulblets was obtained in the bulbscale cultures immersed three times a day for 15 minutes for 5 and 6 weeks (1.83 and 2.07 bulblets per bulbscale, respectively) (Table 1). In the weeks 5th and 6th, we saw a clear tendency to produce more bulblets due to the longer immersion time. At the same time, a preference was noticeable for a longer one-time immersion (RITA 1 × 15: 1.48–1.7 bulblets/scale for a 5- and 6-week pretreatment, respectively) over three shorter immersions (RITA 3 × 5: 0.91–0.97 bulblets/scale for a 5- and 6-week pretreatment, respectively) (Table 1). The frequency of 3 × 15 minutes stimulated the formation of a more significant number of bulblets. However, their weight was lower (0.67 g and 0.5 g) than in analogous combinations immersed in the medium once a day for 15 minutes (0.8 g and 0.75 g) (Table 2).
Table 2
Effects of pretreatment time and immersion frequency on Lilium candidym single bulblet weight [g]. Statistical effect for the source of variation (weeks, frequency, weeks × frequency) for p ≤ 0.001.
| Stage of experiment | Weeks in RITA | Immersion frequency | |||
|---|---|---|---|---|---|
| 3 × 1a | 3 × 5 | 1 × 15 | 3 × 15 | ||
| Pretreatment (liquid medium) | 1 | 0.00ab | 0.00a | 0.00a | 0.00a |
| 2 | 0.00a | 0.00a | 0.00a | 0.00a | |
| 3 | 0.38 ± 0.06ef | 0.64 ± 0.08h | 0.53 ± 0.02g | 0.29 ± 0.04d | |
| 4 | 0.39 ± 0.03f | 0.53 ± 0.02g | 0.69 ± 0.02i | 0.49 ± 0.06g | |
| 5 | 0.33 ± 0.01de | 0.35 ± 0.04ef | 0.80 ± 0.05j | 0.67 ± 0.02hi | |
| 6 | 0.24 ± 0.0c | 0.14 ± 0.0b | 0.75 ± 0.0j | 0.50 ± 0.0e | |
| Regeneration (6 weeks on solid medium) | 0 (control) | 0.46 ± 0.07hi | |||
| 1 | 0.20 ± 0.01a | 0.78 ± 0.07k | 0.40 ± 0.05f–h | 0.34 ± 0.02c–f | |
| 2 | 0.32 ± 0.04c–e | 0.49 ± 0.04i | 0.28 ± 0.02bc | 0.24 ± 0.00ab | |
| 3 | 0.27 ± 0.03bc | 0.25 ± 0.00ab | 0.33 ± 0.02c–e | 0.41 ± 0.03f–h | |
| 4 | 0.28 ± 0.03bc | 0.36 ± 0.02d–g | 0.30 ± 0.02b–d | 0.44 ± 0.02hi | |
| 5 | 0.43 ± 0.04hi | 0.60 ± 0.03j | 0.25 ± 0.02ab | 0.23 ± 0.01ab | |
| 6 | 0.39 ± 0.04e–h | 0.48 ± 0.03i | 0.20 ± 0.02a | 0.40 ± 0.02f–h | |
In the cultures pretreated with the liquid medium for 1–4 weeks at a frequency of 3 × 1, 3 × 5, and 1 × 15 minutes a day, the number of bulblets produced at the regeneration stage was not higher than in the control (Table 1). All bulbscales treated with the liquid medium for six weeks during pretreatment produced a comparable or higher number of bulblets than those on the control scales. The most significant number of bulblets was obtained from the explants treated for 2–6 weeks with the liquid medium at a frequency of 3 × 15 minutes a day (3.23–4.02 bulblets/scale) (Table 1). The weight of these bulblets was not high (0.23–0.44 g). The largest bulblets following six weeks of regeneration were observed in the cultures immersed with a frequency of 3 × 5 minutes a day for one week (0.78 g) and for five weeks (0.6 g) (Table 2).
Bulblet dry weight
During the RITA bioreactor culture (pretreatment), the dry weight of the bulblets decreased systematically from the fourth week, regardless of the immersion frequency. The highest dry matter content of the bulblets was recorded in the fourth week of pretreatment in the cultures immersed one or three times a day for 15 minutes (15.67 and 15.5%, respectively). Similar dry matter content (15.20%) in the third week of pretreatment was shown by bulblets in the combination immersed three times a day for 5 minutes (Table 3). During pretreatment, the dry weight of the bulblets correlated negatively with all tested parameters except single bulblet weight (Table 4).
Table 3
Effects of pretreatment time and immersion frequency on Lilium candidum bulblets dry weight [%]. Statistical effect for the source of variation (weeks, frequency, weeks × frequency) for p ≤ 0.001.
| Stage of experiment | Weeks in RITA | Immersion frequency | |||
|---|---|---|---|---|---|
| 3 × 1a | 3 × 5 | 1 × 15 | 3 × 15 | ||
| Pretreatment (liquid medium) | 1 | − | − | − | − |
| 2 | − | − | − | − | |
| 3 | 13.38 ± 0.49cdb | 15.20 ± 0.57fg | 13.04 ± 0.50bc | 14.47 ± 0.43ef | |
| 4 | 14.07 ± 0.10de | 12.54 ± 0.66bc | 15.67 ± 0.25g | 15.50 ± 0.34g | |
| 5 | 13.05 ± 0.29bc | 11.25 ± 0.49a | 14.05 ± 0.36de | 14.35 ± 0.29e | |
| 6 | 12.81 ± 0.48bc | 10.72 ± 0.41a | 12.42 ± 0.41b | 13.09 ± 0.73bc | |
| Regeneration (6 weeks on solid medium) | 0 (control) | 14.20 ± 0.72i | |||
| 1 | 13.84 ± 0.07i | 9.89 ± 0.25b | 13.93 ± 0.77i | 12.45 ± 0.54e–g | |
| 2 | 12.64 ± 0.36fg | 10.88 ± 0.26c | 15.62 ± 0.28j | 12.12 ± 0.48ef | |
| 3 | 13.12 ± 0.45gh | 10.71 ± 0.33c | 9.56 ± 0.35b | 9.20 ± 0.44ab | |
| 4 | 13.79 ± 0.18hi | 10.83 ± 0.08c | 11.13 ± 0.54cd | 11.05 ± 0.56cd | |
| 5 | 9.55 ± 0.13b | 12.41 ± 0.80e–g | 10.91 ± 0.23c | 10.69 ± 0.17c | |
| 6 | 9.57 ± 0.35b | 8.69 ± 0.04a | 11.74 ± 0.10de | 9.42 ± 0.20b | |
Table 4
Map of the correlations between the biomass growth index and Lilium candidum bulblets parameters in RITA pretreatment.
Increasing the immersion time during pretreatment usually decreased the dry matter content during regeneration (Table 3). The dry weight of the bulblets developing on the solid medium (regeneration) showed a negative correlation with all tested parameters. The dry matter content decreased as the weight, number of bulbs, and amount of sugars they contained increased (Table 5).
Table 5
Map of the correlations between the biomass growth index and Lilium candidum bulblets parameters on solid medium cultured after RITA pretreatment.
Soluble sugars in the bulblets and the liquid medium
Although a decrease in dry matter content was observed during pretreatment, there was an increase in soluble sugars in the bulblet tissues. Regardless of the frequency of immersion, the bulblets pretreated with the liquid medium for a more extended time had a higher content of soluble sugars. Their highest content (565.06 mg/g dw) was recorded in the bulblets grown on the scales during the 6-week pretreatment with the liquid medium at a frequency of 3 × 5 minutes a day (Table 6). All parameters, except dry matter and single bulblet weight, showed a positive correlation with the content of soluble sugars in the bulblets during pretreatment (Table 4).
Table 6
Effects of pretreatment time and immersion frequency on the soluble sugars content in Lilium candidum bulblets [mg/g dw]. Statistical effect for the source of variation (weeks, frequency, weeks × frequency) for p ≤ 0.001.
| Stage of experiment | Weeks in RITA | Immersion frequency | |||
|---|---|---|---|---|---|
| 3 × 1a | 3 × 5 | 1 × 15 | 3 × 15 | ||
| Pretreatment (liquid medium) | 1 | − | − | − | − |
| 2 | − | − | − | − | |
| 3 | 446.77 ± 10.28cdb | 489.88 ± 6.67ef | 414.10 ± 12.94a | 434.32 ± 12.38bc | |
| 4 | 455.71 ± 5.79d | 519.16 ± 13.07gh | 420.26 ± 7.70ab | 485.15 ± 18.25e | |
| 5 | 455.96 ± 9.16d | 528.23 ± 1.64h | 442.31 ± 6.66cd | 506.54 ± 4.32fg | |
| 6 | 492.27 ± 11.84ef | 565.06 ± 3.37i | 507.28 ± 10.73fg | 511.89 ± 12.14gh | |
| Regeneration (6 weeks on solid medium) | 0 (control) | 492.36 ± 11.08l | |||
| 1 | 330.79 ± 1.98c | 341.94 ± 1.55d | 275.81 ± 2.51a | 304.51 ± 4.41b | |
| 2 | 354.56 ± 3.80e | 343.24 ± 2.51d | 305.90 ± 10.46b | 322.41 ± 2.07c | |
| 3 | 376.82 ± 4.19fg | 371.59 ± 2.85f | 375.54 ± 3.77f | 331.17 ± 4.69c | |
| 4 | 378.34 ± 2.85fg | 385.34 ± 9.16gh | 389.49 ± 6.96h | 348.60 ± 5.10de | |
| 5 | 388.90 ± 0.67h | 391.22 ± 3.11h | 409.28 ± 5.10ij | 418.23 ± 4.80j | |
| 6 | 400.90 ± 2.77i | 409.54 ± 4.49ij | 416.55 ± 1.99j | 428.30 ± 8.04k | |
At the stage of regeneration on the solid medium, the lowest content of soluble sugars was characteristic of the bulbscales treated with the liquid medium for the shortest culture time. The longer the culture was treated with the liquid medium during pretreatment (regardless of the immersion frequency), the higher the content of soluble sugars was in its tissues at the end of the 6-week regeneration. The bulblets obtained in the control had a lower or comparable content of soluble sugars than those obtained from the explants cultivated in the bioreactor alone but higher than the bulblets in the cultures transferred from the bioreactor to the solid medium (Table 6). In contrast with pretreatment, the content of soluble sugars in the bulblets on the solid medium (regeneration) showed a weaker correlation with other parameters except the number of bulblets (Table 5).
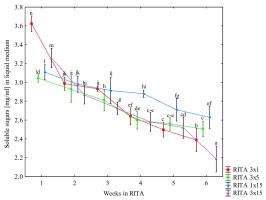
For all tested combinations of immersion frequencies, a gradual decrease in the content of soluble sugars in the liquid medium was observed. Between the 2nd and 5th weeks of pretreatment, it was relatively consistent for all combinations except RITA 1 × 15, which retained the highest sugar content until the last week (Figure 4). This combination was characterized by the lowest content of soluble sugars in the bulblets until the 5th week of pretreatment (Table 6). The most effective sugar absorption from the medium was observed in the RITA 3 × 15 variant in the sixth week of the bioreactor culture. Compared with RITA 3 × 5, which was characterized by the highest content of soluble sugars in bulblets at the end of pretreatment (Table 6), the uptake of these compounds from the medium was, however, lower in the initial weeks of pretreatment (Figure 4).
. Discussion
Short-term explant pretreatment with solutions or media containing growth regulators is a common technique generally employed for promoting the formation of adventitious shoots on explants (D’Onofrio & Morini, 2006; Thomas, 2007) and increasing their regenerative convertibility into shoots (Jahan et al., 2011). Increasing multiplication efficiency can also be achieved through sequential cultivation using cycles in liquid and solid media, where the efficiency depends on the period of exposure to the liquid media (Malik, 2008; Malik et al., 2018). The experiment involved 1- to 6-week pretreatment in a bioreactor with a RITA®; periodic flooding system with various immersion frequencies. The results of our investigation indicated that the duration of pretreatment and immersion frequency in the RITA®; bioreactor shaped regeneration efficiency on the solid medium. A longer pretreatment time (5–6 weeks) resulted in a greater biomass growth index (weight gain) during the regeneration stage.
Periodic contact of the explant tissues with the liquid medium promotes proper mixing of the medium components and optimal aeration (Etienne & Berthouly, 2002), ensuring more uniform culture conditions. In bioreactor vessels with periodic immersion, the culture atmosphere is completely exchanged during culture immersion. This seems to be necessary for the proper development of L. candidum in the liquid medium, as its bulbscales died or showed signs of hyperhydricity in a stationary and shaken liquid medium (Daneshvar Royandazagh, 2019). Better nutrient uptake and aeration of the culture and more frequent air changes in the RITA bioreactor may explain higher bulblet yield (pretreatment in RITA 3 × 15) and larger bulblet size (pretreatment in RITA 3 × 5) in the case of more frequent immersion cycles. On the other hand, a longer duration of apical dominance-disrupting movement may explain the tendency to create a greater number of smaller bulblets when the total time of the rest phase is shorter (RITA 3 × 15 min). A similar decrease in explant weight with increasing time and frequency of contact with a liquid culture medium was observed for Lilium oriental hybrid ‘Casablanca’ (Lian et al., 2003). Reducing the resting time to 45–60 minutes between 15-minute immersions promoted microtuber formation of Chlorophytum borivilianum Sant. and Fernand in the RITA system (Ashraf et al., 2013).
Bulbscales serving as explants have a large surface area in relation to their volume. This facilitates the absorption of nutrients from the medium and weight gain (Langens-Gerrits et al., 2003). Bioreactor cultures, through the use of liquid medium, provide very good access and utilization of these substances (de Klerk, 2012; Ilczuk et al., 2005). This explains the positive effect of a longer overall immersion time on the efficiency of organogenesis in L. candidum bulblets. Compared with solid medium cultures, an increased number of adventitious bulbs in bioreactor cultures was observed in Hippeastrum (Ilczuk et al., 2005; Takayama & Yokokawa, 1996). A more significant number of bulblets with a higher weight provides benefits when the material is acclimatized to ex vitro conditions. Larger bulbs of Leucojum aestivum sprouted faster (Ptak, 2014), and larger bulbs of various species of the Lilium genus emerged faster when sown in the field (Lapiz-Culqui et al., 2022). Smaller bulblets of lilies develop cauline leaves, allowing photosynthesis and increase in size ex vitro at a much lower rate than large bulblets. All bulblets of Lilium ‘Casablanca’ obtained in a bioreactor culture developed cauline leaves after six weeks of acclimatization, which was not the case for all bulblets from a solid medium (Lian et al., 2014).
In bioreactor cultures of bulbous plants (Ilczuk et al., 2005; Ptak, 2014), lower dry matter content is often observed in the tissues of the propagated plant material compared to those obtained on a solid medium. In some types of bioreactors, hyperhydricity may occur (Dewir et al., 2014). Regardless of the frequency of immersion and the length of the pretreatment period, and despite the dry matter content of L. candidum bulblets being lower than in the control, we did not observe hyperhydricity. Ptak’s (2014) study on Leucojum aestivum also did not demonstrate a negative impact of low dry matter content and altered non-functional stomata in plants grown in liquid media on acclimatization. Plants from bioreactor cultures acclimated to ex vitro conditions faster than those obtained on a solid medium.
Bulbs, as storage organs, accumulate significant amounts of sugars in their tissues and absorb them from the culture medium (Langens-Gerrits et al., 2003). In L. candidum bulbscale cultures, an increase in the content of soluble sugars was observed with the extension of pretreatment in the RITA bioreactor. In addition to being a carbon source, soluble sugars also have osmoprotective functions and are involved in defense against stress by influencing cell membranes, hormonal stability, and playing a signaling role (Ahmad et al., 2020). Better availability of liquid medium components (Ilczuk et al., 2005) may affect the activity of cytokinins, which activate invertase and allow for sugar utilization, also promoting tuberization (Sami et al., 2016). Proper provision of the liquid medium is essential to obtain high-quality bulblets that utilize the sugars contained therein throughout the culture period (Lian et al., 2003). Therefore, for longer culture times, replacing or supplementing the medium is beneficial or even required (Lian et al., 2002). The RITA system has been used not only for improving proliferation but also for improving the quality of regenerants (Pérez et al., 2013).
. Conclusions
Pretreatment in the temporary immersion bioreactor system RITA®; significantly affected the weight, number, and parameters of Lilium candidum bulblets. Extending the pretreatment time with a liquid medium (RITA bioreactor) resulted in an increased biomass growth index (weight gain) of the bulblets and their larger number at the regeneration stage. Organogenesis can also be influenced by selecting the frequency of immersion. More bulblets can be obtained by immersing lily scales less frequently but for a longer time (e.g., 1 × 15 min a day), while more frequent immersion (e.g., 3 × 5 min a day) for a shorter time results in a greater increase in biomass. At both stages, longer contact time with the liquid medium increased the content of soluble sugars in bulblets. A more extended pretreatment period resulted in a decrease in the percentage of dry matter in the bulblet tissues, but we did not observe any symptoms of hyperhydricity. Considering our results obtained in the liquid medium, which is particularly useful for L. candidum commercial production, it seems to be the use of three fifteen-minute immersions during the day. It seems justified to cease transferring the material to the solid medium and continue the bioreactor culture of L. candidum bulbscales while replacing the medium with a fresh one. This would allow for additional improvement of micropropagation outcomes of this valuable species. Further studies should be conducted to confirm this thesis.