. Introduction
Polyploidy occurs when cells possess more than two complete sets of chromosomes. This phenomenon plays an important role in plant speciation and evolution in nature and in agriculture (for improvement of popular crops and ornamental plants, such as Allium cepa, Cucumis sativus, Petunia sp., or Vitis sp.) (Touchell et al., 2020). Increased yield, large fruits, and enhanced vigor are some advantages of polyploid plants. Thus, distinguishing plants with various ploidy levels is crucial for plant research and breeding programs (Dirihan et al., 2013). It is also imperative to choose the best method for distinguishing ploidy, because not all established methods will be successful for a particular plant (Cramer, 1999). Genome size has a direct effect on the size of certain cell types (including trichomes and epidermal and stomatal cells). Consequently, genome size affects plant morphological features (Beaulieu et al., 2008). Stomata size and density are correlated with ploidy levels (Beaulieu et al., 2008; Wang et al., 2020). The aforementioned method has been useful for detection of plants with high ploidy, such as Coffea (Mishra, 1997), Stevia (Hata et al., 2001), Pyrus (Kadota & Niimi, 2002), Citrus (Padoan et al., 2013), and Passiflora (Antoniazzi et al., 2018).
Actinidia chinensis var. deliciosa A. Chev. (A. Chev.), known as kiwifruit, is a hexaploid plant (Huang, 2016) of great breeding importance for its fruit (Richardson et al., 2018). Its endosperm can be successfully cultivated in vitro and redifferentiated into plant (Góralski et al., 2005). Studies have thus been conducted on kiwifruit regenerants to confirm their ploidy (Chłosta et al., 2021; Góralski et al., 2005), but detailed analyses of the morphological differences between nonaploid regenerants and hexaploid plants have not been carried out yet. The aim of our study was to verify whether stomatal size is larger and density is smaller in nonaploid than in hexaploid kiwifruit leaves. We examined whether these features could be useful for distinguishing these two groups of plants.
. Material and Methods
For this study, we used materials obtained from commercially available fruit of A. chinensis var. deliciosa ‘Hayward.’ Endosperm tissue was isolated and maintained under conditions in the protocols described by Góralski et al. (2005) and Popielarska-Konieczna et al. (2011), respectively. Briefly, seeds were taken from the fruit after sterilization, the seed coats and embryos were removed, and the endosperms were transferred onto basal Murashige and Skoog (1962) medium (Duchefa) supplemented with 0.5 mg/L thidiazuron (Sigma). For controls, plants were obtained from seeds grown under the conditions described by Popielarska-Konieczna et al. (2011). Each callus line obtained from a particular endosperm explant was individually numbered. Similarly, each regenerant from a specified callus line was distinguished as previously described by Chłosta et al. (2021). The abbreviations 6Cx and 9Cx were used for hexaploid (2C DNA content) and nonaploid plants (derived from endosperm cells with 3C DNA content), respectively. Regenerants and control plants had ploidy predetermined by flow cytometry, according to Chłosta et al. (2021).
Twelve endosperm-derived regenerants (ten with 9Cx and two with 6Cx DNA) and nine 6Cx control plants were selected for stomatal feature measurements (Table 1). The selected acclimatized plants [maintained under the conditions described by Chłosta et al. (2021)] had five to seven fully expanded leaves. The third or fourth expanded leaf from the top of each plant were simultaneously harvested for analyses. Samples were prepared according to previously established protocols (Popielarska-Konieczna et al., 2011). Briefly, leaves were immediately prefixed in 5% buffered glutaraldehyde (0.1 M phosphate buffer, pH 7.2) for 2 hr at room temperature. After dehydration through a graded ethanol series, samples were dried with a CPD system (CO2 critical-point drying), sputter-coated with gold (JEOL JFC-1100 E ion sputtering system), and observed using a scanning electron microscope (JEOL JSM 5410). The abaxial region close to the midrib in the middle of the leaf was observed (Figure 1A,B). Photographs were processed using Corel Photo-Paint X7 software. Number of stomata was determined in three areas, comprising 0.2 mm2 per leaf, and was reported in units of stomata per 1 mm2. One hundred measurements of open stomata (measured as a complex of two guard cells and a pore) width and length per specimen were taken using ImageJ 1.48 software.
Table 1
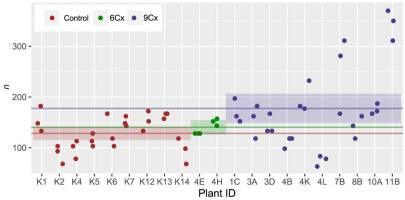
Plants of Actinidia chinensis var. deliciosa chosen for analysis of stomatal traits; seedlings (6Cx control) and regenerated plants (6Cx and 9Cx).
| Group of samples | Plants ID |
|---|---|
| 6Cx control | K1, K2, K4, K5, K6, K7, K12, K13, K14 |
| 6Cx | 4E, 4H |
| 9Cx | 1C, 3A, 3D, 4B, 4K, 4L, 7B, 8B, 10A, 11B |
Figure 1
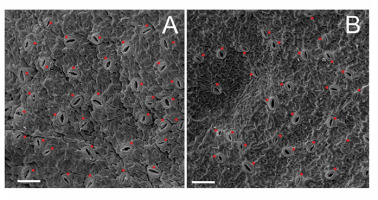
Electronograms of epidermal surfaces with stomata (red dots) from abaxial surfaces of Actinidia chinensis var. deliciosa leaves; (A) endosperm-derived nonaploid 9Cx, and (B) control hexaploid 6Cx. Scale bars = 100 µm.
Statistical analyses were performed using R software (R Core Team, 2018). Three groups of samples (Table 1) were compared for number of stomata per 1 mm2, and stomata length, width, and length/width. Equality of variances between groups was tested using Levene’s test of equality of variance [leveneTest() function]. Because variances were not homogenous (p < 0.05) in all cases, Welch’s heteroscedastic F test (Welch test) was applied. The ggplot2 package (Wickham, 2016) was used to generate Figure 2–Figure 4.
Figure 2
Mean length (A), width (B), and ratio of length to width (C) of the stomata in Actinidia chinensis var. deliciosa seedlings (6Cx control) and endosperm-derived plants (6Cx and 9Cx regenerants); error bars show 95% confidence intervals.
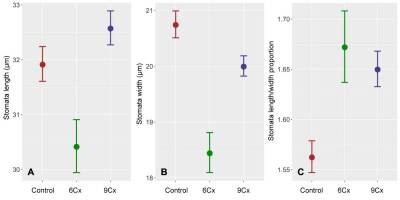
Figure 3
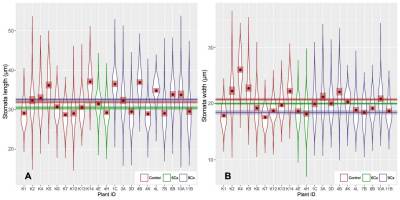
Stomatal length (A) and width (B) in seedlings (6Cx control) and endosperm-derived plants (6Cx and 9Cx regenerants). Graphs show the patterns of distribution of measurements of all analyzed individuals. Horizontal lines accompanied by semitransparent bands in appropriate colors show mean values and 95% confidence intervals (CI) for the measurement sets. Brown dots show the mean values for a given dataset, with 95% confidence intervals marked as semitransparent brown squares.
. Results and Discussion
Preliminary studies (Popielarska-Konieczna & Kleszcz, 2015) suggest visible differences in the size and density of stomata between control plants and regenerants with 6Cx DNA content and those with 9Cx content. Here, all three groups of samples (control 6Cx, 6Cx regenerants, and 9Cx) were significantly different in terms of both the length and width of the stomata calculated for all measurements of length and width for a group (Figure 2A,B, Table S1). Significant differences in guard cell size have been reported for other kiwifruit polyploids (Boase & Hopping, 1995; Przywara et al., 1988). In the present study, the stomata of 9Cx plants were generally longer than those of the control and 6Cx regenerants, but the stomata in the control plants were wider. Means for stomatal dimensions differed significantly between samples, even within the same group of tested plants (Figure 3). Although the means for two samples within a group may differ significantly, those for samples from different groups were in many cases almost the same, and the differences were insignificant (Figure 3B). Spreads between the minimal and maximal values for stomatal measurements overlapped for all samples. Mean stomata length and width proportions (Figure 2C) were the lowest for the control plants and significantly differed from those of the other two groups, which did not significantly differ from each other.
Very small significant differences in stomatal size have been reported for Citrus clementina (Padoan et al., 2013), and the authors suggest that this method could be useful for determining ploidy. Although positive correlations were observed between ploidy and stomatal guard cell length, the overlap of values between groups was too large to permit reliable identifications. By contrast, data from Morus alba (Thomas et al., 2000) and Passiflora edulis (Antoniazzi et al., 2018) permit the use of stomatal cell size to distinguish triploid plants from diploid parents. Our data, combined with the above studies, support the conclusion that although ploidy may coincide with stomata dimensions, the usefulness of measuring stomatal size to determine ploidy is species dependent.
Another frequently proposed method for estimating ploidy is to calculate stomata density. Our three-group analysis (Figure 4, Table S1) revealed that differences in the means were only significant between 6Cx control and 9Cx regenerant groups. The spread in values was very high, especially for 9Cx regenerants. Although stomatal densities measured within a group may vary significantly, they can be similar for samples from different groups (Figure 4). These results disagree with the results of previous studies showing an increase in stomatal length concurrent with an increase in ploidy and a decrease in stomatal density (Antoniazzi et al., 2018; Padoan et al., 2013).
. Limitations of the Study
The results presented indicate some potential correlations, which must be confirmed among larger groups of control plants, 9Cx regenerants, and especially 6Cx regenerants. In the present study, the limited number of hexaploid regenerants did not allow us to consider the possibility of distinguishing 6Cx control plants and 6Cx regenerants.
. Conclusions
Our results suggest a correlation between ploidy and stomatal dimension means and stomatal densities calculated for numerous individuals. In particular, the means of stomatal length and density were higher for nonaploid regenerants. However, the method cannot reliably distinguish individual hexaploid and nonaploid plants owing to significant variation in these values within study groups. Although generally, the mean stomatal length for nonaploids is higher than that for hexaploids, when values for individuals are compared, they may be similar or hexaploid stomata may in some cases be longer than nonaploid ones.