. Introduction
Chrysanthemum × morifolium (Ramat.) Hemsl. is a perennial ornamental plant belonging to the family Asteraceae. It is a highly attractive short-day plant with a wide range of cultivars differing in characteristics such as color, shape and sizes of inflorescences, plant architecture, flowering time, postharvest quality, and biotic and abiotic stress tolerance (J. Pandey et al., 2022; Tymoszuk & Kulus, 2020). Chrysanthemum flowers contain significant amounts of nutritive and biologically active components and are also used in the medical, food, and beverage industries. This species is cultivated worldwide, ranking second in the cut flower trade (after the rose), and provides significant earnings to the producing countries (Su et al., 2019). Due to the popularity and economic importance of chrysanthemum, more research efforts are still needed regarding cultivar breeding and large-scale production of high-quality cuttings (Ali et al., 2023; Tymoszuk & Kulus, 2020).
Propagation of chrysanthemum through in vitro culture has become a viable alternative to conventional propagation methods. Protocols for rapid production of true-to-type and disease-free plants by axillary bud proliferation or shoot-tip culture are especially desired for cuttings production for commercial purposes in horticulture (Waseem et al., 2009). Shoot-tip cultures are also used for rapid clonal propagation, virus elimination, germplasm conservation, or genetic transformation protocols of plants (Nehra & Kartha, 1994). In chrysanthemum micropropagation, shoot-tips are likewise used during the rooting stage. Rhizogenesis is usually induced on a medium supplemented with a suitable auxin (Bhojwani & Dantu, 2013; Christiaens et al., 2019). The most often used auxins in chrysanthemum rooting are indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) or 1-naphthaleneacetic acid (NAA), at concentrations ranging from 0.1 to 2.0 mg ⋅ L−1 (Eisa et al., 2022). Auxins promote cell division and elongation, stimulate root primordium formation, and promote starch hydrolysis and sugar mobilization to the cutting base (Abdallatif et al., 2022; Molassiotis et al., 2004; Thangavelu et al., 2016). Nevertheless, the use of auxins has some limitations, such as the reduction or inactivation of their biological activity by high temperature during medium autoclaving or by light during medium storage and inhibition of root elongation in response to high auxin concentrations (Abdallatif et al., 2022; Khai et al., 2022).
Investigating the possibilities of using nanoparticles, i.e., ZnO NPs, to increase shoot growth and root regeneration of in vitro propagated plants is a relatively new approach (Alizadeh & Dumanoğlu, 2022). Unique optoelectrical, physical, and antimicrobial activities of ZnO NPs offer great potential to enhance the productivity of crops, which was proved in experiments on Brassica oleracea var. italica Plenck (Awan et al., 2021), Linum uitatissimum L. (Sadak & Bakry, 2020), Lycopersicon esculentum Mill. (Faizan et al., 2020), or Perilla frutescens var. crispa f. purpurea (Salachna et al., 2021). Interestingly, zinc has an important function in the synthesis and accumulation of tryptophan, which is a precursor of natural auxin IAA (Li et al., 2021; Sarkhosh et al., 2022). Positive effects of zinc oxide nanoparticles on root formation were proved in Cicer arietinum L. (A. C. Pandey et al., 2010), Zea mays L. (López-Reyes et al., 2022), or Malus domestica Borkh. (Alizadeh & Dumanoğlu, 2022). These suggest that zinc nanoparticles may be used as a substitute for exogenous auxins in plant in vitro cultures. Moreover, Zn is an essential plant micronutrient playing an important role in plant growth, propagation, and yielding by controlling the activity of enzymes and hormones in many integral metabolic processes, as well as regulating the biosynthesis of chlorophyll, proteins, carbohydrates, phenolics, DNA, and RNA (Awan et al., 2021; Pałka et al., 2023).
Silver nanoparticles, as one of the most recognized nanomaterials, have also been used in plant tissue cultures, protection, fertilization, and flower shelf life extension in horticulture, exhibiting a broad spectrum of biological activity and affecting plant growth and quality (Byczyńska et al., 2023). Studies on AgNPs effects on in vitro seed germination, plant propagation, and metabolite production revealed positive and negative aspects, depending on tested concentration or plant species (Tymoszuk, 2021). More in-depth research is needed to understand the intricate complexities involved in nanoparticle action (Parzymies, 2021).
This study aimed to test the influence of zinc oxide submicron particles (ZnO SMPs), zinc oxide nanoparticles (ZnO NPs), and zinc oxide nanoparticles combined with silver nanoparticles (ZnO+Ag NPs), applied at the concentrations of 100, 200, or 400 mg ⋅ L−1, on the in vitro growth of shoots and regeneration of adventitious roots in chrysanthemums ‘UTP Burgundy Gold’ and ‘UTP Pinky Gold’. The proposed approach, assuming simultaneous stimulation of shoot development and root regeneration, would be of practical importance in protocols used in chrysanthemum microcuttings production.
. Material and methods
Characteristics of used nanoparticles
The synthesis of nanostructured ZnO NPs and ZnO+x%Ag NPs in this research included the use of several materials such as zinc acetate dihydrate (Zn(CH3COO)2 ⋅ 2H2O, Avantor Performance Materials Poland S.A., Gliwice, Poland); silver acetate anhydrous (Ag(CH3COO), Chempur, Piekary Śląskie, Poland); ethylene glycol (C2H4(OH)2, Chempur, Piekary Śląskie, Poland); deionized water (H2O) (specific conductance below 0.1 µS ⋅ cm−1). All the chemical substances were analytically pure and used without further purification. Submicron particles of pharmaceutically pure zinc oxide (ZnO SMPs) were purchased from ZM SILESIA SA, Huta Oława, Oława, Poland.
ZnO NPs and ZnO+x%Ag NPs samples were obtained by microwave solvothermal synthesis (Wojnarowicz et al., 2020) using the author’s procedure described in papers (Pokrowiecki et al., 2019; Tymoszuk et al., 2022). The compositions of the precursor solutions can be found in Table S1. Six samples were obtained and named as follows: ZnO NPs 1.5% H2O; ZnO NPs 6% H2O; ZnO+0.1%Ag NPs 1.5% H2O; ZnO+0.1%Ag NPs 6% H2O; ZnO+1%Ag NPs 1.5% H2O and ZnO+1%Ag NPs 6% H2O. Commercial submicron zinc oxide (ZnO SMPs) was used as a reference material.
The testing of the samples was carried out at the Laboratory of Nanostructures (IHPP PAN, Warsaw, Poland), which is accredited with accreditation no. AB 1503. A description of the research procedures used can be found in the paper (Wojnarowicz et al., 2018).
X-ray powder diffraction (XRD) patterns were tested with an X’Pert PRO X-ray diffractometer (CuKα, Panalytical, Almelo, The Netherlands). Morphology was tested using a scanning electron microscope (ULTRA PLUS, ZEISS, Oberkochen, Germany). Skeletal density was examined using a helium pycnometer (AccuPyc II 1340, FoamPyc V1.06, Micromeritics®;, Norcross, GA, USA). The specific surface area was measured by the Brunauer–Emmett–Teller (BET) method (Gemini 2360, V 2.01, Micromeritics®;, Norcross, GA, USA). Zinc and silver content was determined by energy dispersive spectrometry (Quantax 400, Bruker, Billerica, MA, USA). The water content (wt%) of the glycol solution samples was measured using the Karl-Fischer method (Cou-Lo AquaMAX KF, GR Scientific, Bedford, UK).
The average crystallite size (diameter) was obtained using the Scherrer equation. The average particle size (diameter) was calculated from the skeleton density results and the specific surface area (SSA) results. The results of sample characterization can be found in the supplementary materials (Table S2, Table S3, Figure S1). The nanopowder samples obtained by the microwave method were characterized by a uniform size with a homogeneous spherical shape, which was confirmed by SEM results (Figure S2). For samples ZnO SMPs; ZnO NPs 1.5% H2O; ZnO NPs 6% H2O; ZnO+0.1%Ag NPs 1.5% H2O; ZnO+0.1%Ag NPs 6% H2O; ZnO+1%Ag NPs 1.5% H2O; ZnO+1%Ag NPs 6% H2O particle size was 240 nm, 25 nm, 65 nm, 29 nm, 79 nm, 27 nm and 53 nm, respectively.
Chrysanthemum in vitro culture – Establishment, treatments, conditions
Two Chrysanthemum × moriflorium (Ramat.) Hemsl. cultivars: ‘UTP Burgundy Gold’ and ‘UTP Pinky Gold’, were used in the experiment. For in vitro propagation, the modified MS medium (Murashige & Skoog, 1962) was used. The content of calcium and iron in the medium was increased by half. The medium was supplemented with 30 g ⋅ L−1 sucrose and solidified with 8 g ⋅ L−1 Plant Propagation LAB-AGARTM (BIOCORP, Warsaw, Poland). The medium pH was adjusted to 5.8 after adding all of the nutrients. Next, 40 mL of the medium was poured into 350 mL glass jars sealed with plastic and autoclaved at 105 kPa and 121 °C for 20 min.
Shoot fragments containing an apical bud and four underlying nodes, dissected from plantlets cloned previously with the single-node method on the modified MS medium without plant growth regulators (PGRs), were used as explants. Four explants were placed in a vertical position per each culture jar. Each experimental object consisted of four jars (16 shoot fragments in total). Immediately after the inoculation in the medium, the explants were treated with zinc oxide submicron particles suspension (ZnO SMPs) or with nanoparticles suspensions: ZnO NPs 1.5% H2O; ZnO NPs 6% H2O; ZnO+0.1%Ag NPs 1.5% H2O; ZnO+0.1%Ag NPs 6% H2O; ZnO+1%Ag NPs 1.5% H2O; ZnO+1%Ag NPs 6% H2O, at the concentration of 100, 200, or 400 mg ⋅ L−1. The prepared suspensions were sterilized in an autoclave, and, before application on explants, placed for 30 minutes in the Elmasonic S80(H) Ultrasonic Cleaner with the ultrasonic frequency of 37 kHz and the effective ultrasonic power of 150 W (Elma Schmidbauer GmbH, Singen, Germany) to achieve better dispersion of particles. Suspensions were poured onto the culture medium with an automatic pipette with a sterile tip, 2 mL of each colloid at each tested concentration per culture jar. Explants inoculated on the medium without SMPs, NPs, or PGRs were used as the control. In order to evaluate the influence of the tested nanoparticles on the formation of adventitious roots, non-treated with SMPs or NPs explants were also cultivated on the modified MS medium fortified with 2 mg ⋅ L−1 indole-3-acetic acid (IAA) (Sigma-Aldrich, St. Louis, MO, USA) to stimulate rhizogenesis (standard protocol for chrysanthemum in vitro rooting and cutting production used in our previous experiments; Tymoszuk & Kulus, 2020; Tymoszuk & Miler, 2019).
In vitro cultures were maintained in the growth room at the temperature set at 23 ± 1 °C, under 16/8 h light/dark photoperiod, with the use of the Philips TLD 36W/54 fluorescent lamps emitting cool daylight (Koninklijke Philips Electronics N.V., Eindhoven, the Netherlands). The photosynthetic photon flux density was set at 35 µmol m−2 ⋅ s−1.
After three weeks, whole plantlets with regenerated roots were taken out of the culture jars, and biometric data, such as the number of leaves (NL), shoot fresh weight (mg) (SFW), shoot dry weight (mg) (SDW), root system fresh weight (mg) (RSFW), root system dry weight (mg) (RSDW), were collected. Subsequently, leaves and root systems were scanned (Epson STD4800 scanner, USA) and subjected to further biometric measurements. Afterward, the obtained pictures were analyzed to measure the leaf area (LA) (cm2), leaf perimeter (LP) (cm), maximal leaf vertical length (MLVL) (cm), and maximal leaf horizontal width (MLHW) (cm), using the imaging software WinFOLIATM (Reagen Instruments, Quebec, Canada), as well as the total length of the root system (TLR) (cm), root system area (RSA) (cm2), root diameter (RD) (mm), root system volume (RSV) (cm3), number of root tips (NRT), and number of root forks (NRF) using the imaging software WinRHIZOTM (Reagen Instruments, Quebec, Canada).
Statistical analysis
The experiment was set up in a completely randomized design. Each experimental object consisted of 16 repetitions (one plantlet was considered as one repetition; four plantlets were cultured per jar, four jars were used in each experimental object). The normality of the distributions of the observed traits was tested using Shapiro-Wilk’s normality test (Shapiro & Wilk, 1965) to verify whether the analysis of variance (ANOVA) met the assumption that the ANOVA model residuals followed a normal distribution. Bartlett’s test was applied to test the homogeneity of variance. Box’s M test was used to verify multivariate normality and homogeneity of variance-covariance matrices. Multivariate analysis of variance (MANOVA) was carried out to determine the multivariate effects of treatment, cultivar, and treatment × cultivar interaction. Two-way analyses of variance (ANOVA) were carried out to determine the effects of treatment, cultivar, and treatment × cultivar interaction on the variability of the observed traits. The mean values and standard deviations of traits were calculated. Moreover, Fisher’s least significant differences (LSDs), at the 0.05 level, were calculated and on this basis, homogeneous groups were determined. The relationships between observed traits were estimated using Pearson’s linear correlation coefficients. Relationships of the observed traits were presented in a heatmap. The comparisons between particular levels of the analyzed treatments were tested using the two-sample t-test for equal means for all the observed traits. To account for multiple testing, we used the Bonferroni correction. The results were also analyzed using multivariate methods. The GenStat v. 22 statistical software package (VSN International, 2022) was used for the analyses.
. Results
Detailed analysis of leaf number and biomass of plantlets
The experimental plantlets were of high quality, with a fully developed stem, leaves, and root system. No toxic effects resulting from the application of the tested SMPs and NPs were found. Nevertheless, the biometric parameters of shoots and root systems differed among individual experimental treatments with SMPs and NPs depending on the tested cultivar (Figure 1).
Figure 1
Chrysanthemum × morifolium ‘UTP Burgundy Gold’ and ‘UTP Pinky Gold’ plantlets developed in vitro in shoot-tip culture after three weeks on the modified MS medium, selected experimental treatments; bar = 1 cm.

According to the multivariate test results, both the cultivar and the applied experimental treatment with SMPs and NPs significantly affected the values of a number of leaves, shoot fresh weight, shoot dry weight, root system fresh weight, and root system dry weight analyzed jointly. Significant differences were found for cultivar × treatment interaction (Table S4).
The detailed results of univariate ANOVA tests showing independent main effects and interactions for number of leaves, shoot fresh and dry weights, root system fresh and dry weights are presented in Table 1. Moreover, the presented density plots graphs show in detail the distribution of the values of tested traits (Figure 2).
Table 1
Number of leaves, shoot and root system fresh and dry weights of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment.
Figure 2
Density plots for the number of leaves, shoot and root system fresh and dry weights of plantlets in the tested cultivars of Chrysanthemum × morifolium (NL – number of leaves; SFW – shoot fresh weight (mg); SDW – shoot dry weight (mg); RSFW – root system fresh weight (mg); RSDW – root system dry weight (mg)).
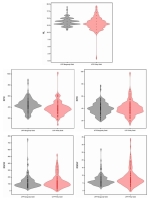
Generally, the cultivar ‘UTP Burgundy Gold’ formed shoots with higher shoot fresh weight (439.1 mg) and higher number of leaves (13.76) compared to the cultivar ‘UTP Pinky Gold’ (shoot fresh weight 375 mg; number of leaves 13.20). Interestingly, ‘UTP Pinky Gold’ roots had a higher dry weight (8.08 mg) than ‘UTP Burgundy Gold’ roots (6.77 mg). The average number of leaves ranged from 11.83 in the control treatment to 15.17 for the ZnO SMPs (400) application, irrespective of the cultivar. Shoots produced on the medium with ZnO+1%Ag NPs 6% H2O (400) had the highest fresh weight (500.3 mg), whereas shoots from the treatment with ZnO SMPs (100) had the highest dry weight (46.65 mg). The lowest shoot fresh and dry weights were reported for ZnO+1%Ag NPs 1.5% H2O (400). Considering the observed values of shoot fresh and dry weights, most often, the use of the tested SMPs and NPs gave results comparable to those obtained after the application of auxin IAA but higher than for the control. Root systems regenerated on the medium with auxin IAA had the highest fresh and dry weights (361.8 mg and 19.31 mg, respectively). The biomass of root systems formed on media with ZnO SMPs or ZnO NPs (without Ag NPs) were higher than those on the control medium, except the treatment with ZnO NPs 1.5% H2O (400), which effect was statistically the same as for control. On the contrary, root systems formed on shoots treated with materials samples containing Ag NPs were characterized by the lowest fresh and dry weights, especially when ZnO+0.1%Ag NPs 6% H2O; ZnO+1%Ag NPs 1.5% H2O; and ZnO+1%Ag NPs 6% H2O were used at the highest tested concentration of 400 mg ⋅ L−1.
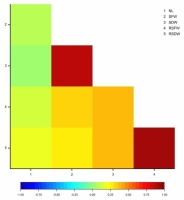
Statistically important, very strong and positive correlations were found between shoot fresh and dry weights (r = 0.8880) and root system fresh and dry weights (r = 0.9312). Weak positive correlations were reported between the number of leaves and root system fresh weight (r = 0.1477), number of leaves and root system dry weight (r = 0.1860), as well as shoot fresh weight and root system dry weight (r = 0.2931), whereas positive moderate correlations were identified between shoot fresh weight and root system fresh weight (r = 0.3391), shoot dry weight and root system fresh weight (r = 0.3983), shoot dry weight and root system dry weight (r = 0.3900). Surprisingly, correlations between number of leaves and shoot fresh and dry weights were not significant (Figure 3).
Figure 3
Heatmap for Pearson’s correlation coefficients between the number of leaves, shoot and root system fresh and dry weights of Chrysanthemum × morifolium plantlets (NL – number of leaves; SFW – shoot fresh weight; SDW – shoot dry weight; RSFW – root system fresh weight; RSDW – root system dry weight).
According to the results of the contrasts analysis, significant differences were found between tested experimental treatments regarding the number of leaves, shoot fresh and dry weights, and root system fresh and dry weights (Table 2). All treatments with ZnO SMPs, ZnO NPs, and ZnO+Ag NPs stimulated more effectively the formation of new leaves on chrysanthemum shoots compared to the control. The application of ZnO SMPs was superior compared to all ZnO+Ag NPs treatments regarding the increases in shoot fresh and dry weights, as well as root system fresh and dry weights. Moreover, all treatments with ZnO NPs outperformed ZnO+Ag NPs treatments considering root system fresh and dry weights.
Table 2
Results of contrasts analysis for the number of leaves, shoot and root system fresh and dry weights of Chrysanthemum × morifolium plantlets – detailed comparison of experimental treatments, independently and depending on the cultivar (NL – number of leaves; SFW – shoot fresh weight; SDW – shoot dry weight; RSFW – root system fresh weight; RSDW – root system dry weight).
Detailed analysis of leaf architecture parameters
The multivariate test results proved that cultivar and experimental treatment significantly affected the values of all four leaf architecture parameters of chrysanthemum plantlets (leaf area, leaf perimeter, maximal leaf vertical length, maximal leaf horizontal width) analyzed jointly. Significant differences were also revealed for cultivar × treatment interaction (Table S5).
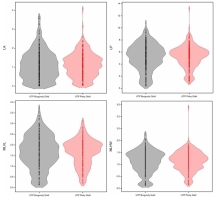
According to the ANOVA results, no significant differences in the values of the analyzed leaf architecture parameters were reported depending on the cultivar (Table 3). Nevertheless, parameters such as leaf area, leaf perimeter, and maximal leaf horizontal width were highly affected by the applied experimental treatments, with the highest values reported for ZnO SMPs (100) and (200); ZnO NPs 6% H2O (100), and ZnO+1%Ag NPs 1.5% H2O (100). Comparing, the control plantlets developed leaves with lower area, perimeter, and maximal horizontal width. The lowest values of the analyzed leaf architecture parameters were found for the treatment with IAA and ZnO NPs 1.5% H2O (400). Additionally, density plot graphs presenting the distribution of the tested leaf traits are shown in Figure 4.
Table 3
Leaf architecture parameters of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment.
Figure 4
Density plots for the leaf architecture parameters in the tested cultivars of Chrysanthemum × morifolium (LA – leaf area (cm2); LP – leaf perimeter (cm); MLVL – maximal leaf vertical length (cm); MLHW – maximal leaf horizontal width (cm)).
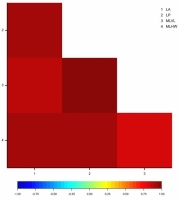
The analysis of linear correlation coefficients indicated highly significant and positive correlations between all the determined leaf parameters in the tested cultivars. Very strong correlations were found between leaf perimeter and maximal leaf vertical length (r = 0.9589), leaf area and leaf perimeter (r = 0.9399), leaf perimeter and maximal leaf horizontal width (r = 0.9398), leaf area and maximal leaf horizontal width (r = 0.9385), leaf area and maximal leaf vertical length (r = 0.8962), as well as maximal leaf vertical length and maximal leaf horizontal width (r = 0.8469) (Figure 5).
Figure 5
Heatmap for Pearson’s correlation coefficients between the leaf architecture parameters of Chrysanthemum × morifolium plantlets (LA – leaf area; LP – leaf perimeter; MLVL – maximal leaf vertical length; MLHW – maximal leaf horizontal width).
The performed contrast analysis revealed significant differences in the ‘UTP Pinky Gold’ cultivar but not in the chrysanthemum ‘UTP Burgundy Gold’ (Table 4). Considering all the analyzed leaf architecture parameters, irrespective of the cultivar as well as in regard to ‘UTP Pinky Gold’ cultivar only, ZnO SMPs were more effective than ZnO NPs 1.5% H2O; ZnO SMPs than ZnO+0.1%Ag NPs 6% H2O; and ZnO NPs 6% H2O than ZnO+0.1%Ag NPs 6% H2O. Interestingly, ZnO SMPs and ZnO NPs 6% H2O were superior compared to the control in terms of increasing the values of leaf area, leaf perimeter, and maximal leaf horizontal width. Moreover, in ‘UTP Pinky Gold,’ the treatment with ZnO NPs 6% H2O vs. ZnO NPs 1.5% H2O, and ZnO+1%Ag NPs 1.5% H2O vs. ZnO NPs 1.5% H2O stimulated more effectively the leaf development.
Table 4
Results of contrasts analysis for the leaf architecture parameters of Chrysanthemum × morifolium plantlets – detailed comparison of experimental treatments, independently and depending on the cultivar (LA – leaf area; LP – leaf perimeter; MLVL – maximal leaf vertical length; MLHW – maximal leaf horizontal width).
Detailed analysis of root systems architecture parameters
The results of MANOVA for root system parameters (total length of the root system, root system area, root diameter, root system volume, number of root tips, number of root forks) analyzed jointly are presented in Table S6. Significant effects of the main experimental factors (cultivar, treatment), as well as their interaction (cultivar × treatment), were proved.
The ANOVA analysis revealed significant effects of the applied treatments on the variability of the analyzed root system architecture parameters. A significant effect of the cultivar was reported for the total length of the root system, root diameter, and number of root forks. ‘UTP Burgundy Gold’ developed root systems with higher total length (37.09 cm) compared to ‘UTP Pinky Gold’ (29.35 cm). Nevertheless, the roots of ‘UTP Pinky Gold’ had a higher diameter (0.542 mm) than ‘UTP Burgundy Gold’ roots (0.456 mm). A number of root tips was higher in ‘UTP Burgundy Gold.’ Significant cultivar × treatment interactions were found for root diameter (Table 5, Table 6).
Table 5
Root system architecture parameters of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment – part 1.
Table 6
Root system architecture parameters of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment – part 2.
Auxin IAA stimulated intensive root elongation (57.18 cm). The total length of the root system was also high for treatment with ZnO NPs 6% H2O (100) (42.60 cm), while for ZnO+0.1%Ag NPs 6% H2O (400) amounted only to 21.02 cm. Most often, when the tested SMPs and NPs were applied at the highest concentration of 400 mg ⋅ L−1, the inhibition of root elongation was observed. In ‘UTP Burgundy Gold’, total lengths of the root system were 24.52 and 56.75 cm, for ZnO+1%Ag NPs 1.5% H2O (400) and IAA, respectively, whereas in ‘UTP Pinky Gold’, 16.57 and 57.62 cm, for ZnO NPs 1.5% H2O (400) and IAA, respectively (Table 5, Figure 6).
Figure 6
Density plots for the root system architecture parameters of plantlets in the tested cultivars of Chrysanthemum × morifolium (TLR – total length of the root system (cm); RSA – root system area (cm2); RD – root diameter (mm); RSV root system volume (cm3); NRT – number of root tips; NRD – number of root forks).
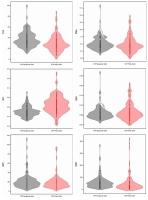
Root system area ranged from 3.32 to 10.47 and from 2.97 to 10.12 cm2 in ‘UTP Burgundy Gold’ and ‘UTP Pinky Gold’, respectively. The highest root system area values were found for IAA treatment (10.29 cm2), while the lowest for ZnO+0.1%Ag NPs 6% H2O (400) (3.15 cm2). The root system area values obtained for all tested SMPs and NPs were statistically comparable to the control treatment (Table 5, Figure 6).
Roots regenerated on medium with IAA, ZnO SMPs (400), ZnO NPs 1.5% H2O (400), and ZnO NPs 6%H2O (400) produced the highest diameter (0.533–0.575 mm). Considering cultivar × treatment interactions, the highest root diameter (0.659 mm) was found for ‘UTP Pinky Gold’ treated with ZnO NPs 6%H2O (400), while ‘UTP Burgundy Gold’ roots developed on medium with ZnO+0.1%Ag NPs 6% H2O (100) had the lowest value of diameter (0.419 mm). Root system volume was mostly affected by the IAA treatment (0.148 cm3). No differences were found between tested SMPs and NPs and control regarding root system volume (Table 5, Figure 6).
A number of root tips ranged from 14.67 to 58.67 in ‘UTP Burgundy Gold,’ and from 12.67 to 66.83 in ‘UTP Pinky Gold.’ The best response regarding the increase in number of root tips was found for IAA (62.75), whereas roots from ZnO+1%Ag NPs 1.5% H2O (400) treatment formed only 13.83 tips. The decrease in a number of root forks resulting from SMPs and NPs applications was observed compared to IAA (Table 6, Figure 6).
Very strong and positive correlations were identified between total length of the root system and root system area (0.9436), total length of the root system and root system volume (0.8237), total length of the root system and number of root forks (0.7708), root system area and root system volume (0.9642), root system area and number of root forks (0.8316), as well as root system volume and number of root forks (0.8117). Correlations between total length of the root system and number of root tips (0.6260), root system area and number of root tips (0.6641), root diameter and root system volume (0.5323), root system volume and number of root tips (0.6403), number of root tips and number of root forks (0.6997) were strong, whereas those between root diameter and number of root tips (0.1756), root diameter and number of root forks (0.2597) were very weak. The correlation between root system area and root diameter (0.3254) was moderate. No significant correlation was found between the total length of the root system and root diameter (Figure 7).
Figure 7
Heatmap for Pearson’s correlation coefficients between the root system architecture parameters of Chrysanthemum × morifolium plantlets (TLR – total length of the root system; RSA – root system area; RD – root diameter; RSV root system volume; NRT – number of root tips; NRD – number of root forks).
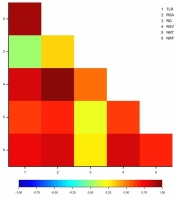
According to contrast analysis, ZnO SMPs, ZnO NPs 1.5% H2O, and ZnO NPs 6% H2O stimulated the regeneration of roots with higher diameter compared to the control (Table 7). All treatments with the use of ZnO NPs and ZnO+Ag NPs were not as effective as the control in regard to increases in the number of root forks. Root diameter and root system volume were higher in ZnO SMPs treatments compared to all ZnO+Ag NPs treatments. Roots regenerated in the presence of ZnO NPs had higher diameters compared to roots formed under the influence of all ZnO+Ag NPs. Moreover, root system volume was higher for ZnO NPs 6% H2O compared to all ZnO+Ag NPs treatments.
Table 7
Results of contrasts analysis for root system architecture parameters of Chrysanthemum × morifolium plantlets – detailed comparison of experimental treatments, independently and depending on the cultivar (TLR – total length of the root system; RSA – root system area; RD – root diameter; RSV root system volume; NRT – number of root tips; NRD – number of root forks).
. Discussion
Nanoparticles, due to their unique properties, have attracted attention in plant micropropagation as potential medium additives, increasing propagation efficiency and improving plantlet quality. Plant tissue cultures provide an ideal environment to study the effect of NPs on plants. The sterile environment and the chemically defined culture medium composition avoid most of the external factors which could influence NPs’ interaction with plants (Radi et al., 2018). Positive aspects of different NPs applications in plant in vitro cultures have been reported; most often for the elimination of microbial contaminations, stimulation of seed germination and seedling development, induction of caulogenesis, adventitious organogenesis, somatic embryogenesis, or metabolite production (Álvarez et al., 2019). Nevertheless, nanoparticles may also negatively influence plant metabolism, growth, and development (Tymoszuk, 2021). NPs are assumed to modify the structural components of cellular membranes and macromolecules, influence cell division and defense systems, and interfere with plant physiological and biochemical processes by altering gene expression (Khan et al., 2021). The interaction between nanoparticles and plant cells depends on the chemical constituting, sizes, shapes, surface covering, reactivity, concentrations, and mode of nanoparticle application, as well as the genotype, age, and developmental phase of a plant (Aly et al., 2023). Our results are in line with other studies revealing both advantages and disadvantages of NPs interactions with plants.
We proved that the application of ZnO SMPs, ZnO NPs and ZnO+Ag NPs, similarly to IAA, stimulated the formation of new leaves on chrysanthemum shoots. Generally, the SMPs- and NPs-treated plantlets formed the most developed leaves with the highest area, perimeter and horizontal width. Additionally, the use of the tested SMPs and NPs increased most often the shoot biomass, and respectively, the highest shoot fresh and dry weights were stated for 400 mg ⋅ L−1 ZnO+1%Ag NPs 6% H2O and 100 mg ⋅ L−1 ZnO SMPs, respectively. Similarly, the addition of 2–18 mg ⋅ L−1 ZnO NPs to medium increased the number of fresh and dry weights of Olea purpurea L. axillary shoots, improving the efficiency of micropropagation (Regni et al., 2022). ZnO NPs, applied at concentrations of 50, 100, and 200 mg ⋅ L−1, positively influenced plant length, leaf length, leaf number, and root length in Spinacia oleracea L. (Aly et al., 2023). The twofold increase in axillary shoot multiplication rate with simultaneous elevation in carbohydrates, total soluble proteins and free amino acids contents was reported in Phoenix dactylifera (L.) Mill. cultures on medium with 150 mg ⋅ L−1 ZnO NPs. According to the authors, the observed positive effects could be attributed to the Zn role in the enhancement of nutrient absorption from the medium as well as Zn influence on the activities of enzymes controlling the metabolism of carbohydrates, proteins, maintenance of the cellular membrane integrity, and regulation of auxin synthesis (Awad et al., 2020). Likewise, in another in vitro study on P. dactylifera, the ZnO NPs treatment increased the content of elements like N, P, K, S, and the content of the endogenous IAA in treated shoots, which in turn stimulated more intensive shoots multiplication and rooting (Al-Mayahi, 2021). We assume that increases in biometric parameters of chrysanthemum shoots reported in our study are strongly related to the positive influence of zinc on physiological and growth processes, confirming the high importance of zinc as a micronutrient for plants.
Treatment with ZnO NPs resulted in 1.4-fold higher zinc content in Punica granatum L. calli, as compared to ZnO microparticles (Radi et al., 2018). In Nicotiana tabacum L. callus tissue, the accumulation of zinc ions was also higher in ZnO NPs treatment compared to ZnO microparticles, which could be the result of the faster release of zinc ions from nanoparticles. On the other hand, nanoparticles may aggregate over time in an aqueous phase, due to their small size and high surface energy, to micro-size particles and undergo morphological changes leading to their lower dissolution and, finally, similar level of overall ion release from nano- and microforms (Mazaheri-Tirani & Dayani, 2020). This may explain the positive results observed in our study for both ZnO NPs and ZnO SMPs, and is additionally consistent with our earlier findings reported for Allium cepa L. (Tymoszuk & Wojnarowicz, 2020).
Interestingly, the application of ZnO SMPs outperformed ZnO+Ag NPs in terms of shoot fresh and dry weights. Moreover, ZnO SMPs and ZnO NPs resulted in higher fresh and dry root system weights as compared to ZnO+Ag NPs. The application of ZnO+Ag NPs decreased most often the parameters of the root system. Interestingly, as reported by other researchers (Stepanova & Alonso, 2019; Strader et al., 2009), ethylene stimulates the synthesis of IAA, and IAA stimulates ethylene synthesis. Some aspects of auxin response require ethylene response, and some aspects of ethylene response require auxin response. On the other hand, Ag ions are thought to occupy the copper binding side of ethylene receptors and inhibit ethylene response, which finally may lead to rooting inhibition. On the contrary, positive effects of Ag NPs were reported in Phoenix dactylifera (L.) Mill. in vitro cultures (Elsayh et al., 2022). When 3 ml ⋅ L−1 Ag NPs were added to the medium at the multiplication stage, the longest shoots with the highest number of leaves were obtained. The best medium for root formation also contained 3 ml ⋅ L−1 Ag NPs and resulted in the highest rooting percentage (85.4%), root number (8.4), and root length (6.3 cm). Stimulatory effect of combined auxin–AgNPs treatments (AgNPs–IAA or AgNPs–IBA) compared to auxin treatments alone on the rooting efficiency and parameters of root systems were reported in Psidium guajava L. (Abdallatif et al., 2022). Nevertheless, in our previous experiment, Gerbera jamesonii Bolus ex. Hook and Ch. morifolium shoots treated with 10 and 30 ml ⋅ L−1 Ag NPs regenerated fewer roots, which were characterized by the lower area and diameter compared to the control (Tymoszuk & Miler, 2019). The observed differences in rhizogenesis stimulation or inhibition may result from Ag NPs concentration, as well as from plant genotype specificity. Particular species and even cultivars within a species present unique patterns of response to NPs treatment (Nalci et al., 2019; Tymoszuk, 2021). Moreover, species Ch. morifolium demonstrates high sensitivity and variability at the biochemical and genetic level in response to Ag NPs treatment, which can significantly limit regeneration efficiency (Tymoszuk & Kulus, 2020). This sensitivity was even observed at low concentrations of Ag NPs (0.1% and 1%) tested in the present study. Similarly, in our previous experiment, ZnO+Ag NPs deteriorated the efficiency of adventitious shoot regeneration in Ch. morifolium as compared to ZnO SMPs and ZnO NPs (Tymoszuk et al., 2022). It can be concluded that silver nanoparticles are more toxic to chrysanthemum plants than zinc oxide submicron and nanoparticles.
Although the applied SMPs and NPs treatments in our study induced rhizogenesis, the auxin IAA was most effective in the stimulation of increases in root system fresh and dry weights, length, area, and volume, as well as root diameter and the number of root tips and forks. However, the IAA supplementation deteriorated the leaf architecture parameters.
Auxins play a fundamental role in orchestrating the final root system architecture, being a component of endogenous developmental programs, and mediating environmental stimuli (Overvoorde et al., 2010). The most well-characterized auxin-associated processes are a dose-dependent increase in the length of root hairs, bimodal effect of auxin concentration on primary root length, dose-dependent increase in the number of lateral root primordia, stimulation of root thickening, increasing the nutrient and mineral uptake, to finally promote the overall plant elongation growth and development. The IAA is the most common plant hormone of the auxin class. Both tryptophan (Trp)-dependent and Trp-independent IAA biosynthetic pathways were revealed in plants. The majority of previous studies on the IAA biosynthesis described four Trp-dependent pathways (indole-3-acetamide (IAM), indole-3-pyruvic acid (IPA), tryptamine (TRA), and indole-3-acetaldoxime) (Fu et al., 2015).
The increase in the IAA accumulation resulting from ZnO NPs application reported in other studies may be due to the zinc contribution to the biosynthesis of amino acid tryptophan, which is the initiator in the IAA biosynthesis. However, at high concentrations of ZnO NPs, oxidative stress may occur in plant cells, and in turn, the elevated peroxide activity may limit the IAA biosynthesis (Al-Mayahi, 2021). Alizadeh and Dumanoğlu (2022) performed a study on the effects of IAA, IBA, ZnO NPs, and ZnO NPs loaded with IAA (ZnO NPs–IAA) or IBA (ZnO NPs–IBA) on the in vitro rooting of Malus domestica Borkh. microcuttings, and found out that the share of rooted plantlets was 1.5-fold higher in ZnO NPs-IBA compared to IBA, and 1.9-fold higher in ZnO NPs-IAA compared to IAA. However, no rooting was reported for ZnO NPs treatment without auxin, which according to the authors, could result from relatively low tested concentrations of ZnO NPs (1–6 ml ⋅ L−1). Interestingly, in G. jamesonii, selenium nanoparticles added to the medium at the concentration of 0.7, 1.0, and 1.5 ml ⋅ L−1 gave similar results to treatment with IBA regarding rooting efficiency. The results revealed a Se NPs-induced increase in auxin content. Moreover, positive effects of Se NPs were also reported for leaf length, plantlet length, and plantlet biomass (Khai et al., 2022).
In the present study, SMPs and NPs suspensions were poured on the surface of the medium and were not added to the medium during its preparation. We wanted to ensure proper particle dispersion before their application on explants and direct contact between explants and particles, as well as avoid particle sedimentation and changes in their properties arising from interactions with different medium components. Possibly, the obtained results for plantlet development would be different, if particles were inside the medium. As was also reported in other studies, the method of nanoparticle application considerably affects plant responses (Aly et al., 2023; Tymoszuk et al., 2022).
Root thickening can be a physiological response to relieve physical stress on the root apex under conditions of mechanical resistance, as well as a response to the chemical toxicity of dissolved ions. Both in Lactuca sativa L. and Daucus carota L. subsp. sativus, the dose-dependent increase in root diameter occurred uniquely with increasing CuO NPs concentration, whereas CuCl2 treatment only decreased root length (Margenot et al., 2018). Nonetheless, in our study, the highest results for root diameter were noted for the medium with IAA, and interestingly, roots regenerated in response to ZnO SMPs and ZnO NPs treatments had generally higher diameter than control and ZnO+Ag NPs-treated roots. It seems, therefore, that the increase in root diameter was generally one of the parameters of the well-developed root system. Interestingly, we observed that the NP-treated root systems formed significantly fewer root forks compared to the control, which clearly indicated the specific architecture of chrysanthemum roots forming in the presence of NPs. Similarly, in Zea mays L., the application of cerium oxide nanoparticles resulted in a decrease in the number of root forks (Ayub et al., 2023).
. Conclusions
Our study demonstrates the characteristics and influence of diversified ZnO SMPs, ZnO NPs, and ZnO+Ag NPs samples on the growth and architecture of chrysanthemum plantlets developing in shoot-tip culture. We performed extensive biometric and statistical analysis to point out the most prominent treatments for the improvement of chrysanthemum micropropagation. We proved that the specific treatments with tested SMPs and NPs may ameliorate shoot and/or root parameters as against the control medium and auxin application. Generally, plantlets treated with SMPs and NPs formed the most developed leaves, especially when 100 and 200 mg ⋅ L−1 ZnO SMPs, 100 mg ⋅ L−1 ZnO NPs 1.5% H2O, and 100 mg ⋅ L−1 ZnO+1%Ag NPs 1.5% H2O were applied. Additionally, the highest shoot fresh and dry weights were stated for 400 mg ⋅ L−1 ZnO+1%Ag NPs 6% H2O and 100 mg ⋅ L−1 ZnO SMPs, respectively. The highest values of root system fresh and dry weights were reported for auxin IAA, whereas the lowest for ZnO+0.1%Ag NPs 6% H2O, ZnO+1%Ag NPs 1.5% H2O, and ZnO+1%Ag NPs 6% H2O applied at the highest tested concentration of 400 mg ⋅ L−1. Auxin treatment also resulted in the highest parameters of the root system architecture. Interestingly, ZnO SMPs and ZnO NPs increased most often parameters of root system as against ZnO+Ag NPs. This is an innovative approach combining the achievements of nanotechnology and biotechnology, both scientifically and practically. Media supplemented with the tested SMPs and NPs may be an alternative to medium with auxin or standard MS medium during chrysanthemum micropropagation, ensuring the production of plantlets with modified biometric parameters. Future studies should be extended to assess the simultaneous effect of zinc oxide SMPs and NPs and reduced auxin concentration on the growth and development of chrysanthemum plantlets.
. Supplementary material
The following supplementary material is available for this article:
Table S1. Composition of the precursor solution used for synthesis.
Table S2. Characteristics of samples.
Table S3. Results of the analysis of the chemical composition of samples. Energy-dispersive spectrometry (EDS) was used for the analysis.
Table S4. Results of multivariate analysis of variance (MANOVA) for the number of leaves, shoot and root system fresh and dry weights of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment.
Table S5. Results of multivariate analysis of variance (MANOVA) for the leaf architecture parameters of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment.
Table S6. Results of multivariate analysis of variance (MANOVA) for the root system architecture parameters of Chrysanthemum × morifolium plantlets, depending on the cultivar and experimental treatment.
Figure S1. X-ray diffraction patterns of samples.
Figure S2. SEM images of samples: (A, B) ZnO SMPs; (C, D) ZnO NPs 1.5% H2O; (E, F) ZnO NPs 6% H2O; (G, H) ZnO+0.1%Ag NPs 1.5% H2O; (I, J) ZnO+0.1%Ag NPs 6% H2O; (K, L) ZnO+1%Ag NPs 1.5% H2O; (M, Ns) ZnO+1%Ag NPs 6% H2O images taken with the immersion lens detector.
Data availability
The datasets generated during and/or analyzed during the current study are available in the RepOD repository [Tymoszuk, Alicja, 2023, “Study on zinc oxide and silver effects on architecture of chrysanthemum plantlets propagated in shoot-tip culture”, https://doi.org/10.18150/G4XUK1, RepOD, V1].


