Introduction
Climatic changes, which result in the elevation of the sea and ocean levels as well as more aggressive abrasion of seashores (Jakusik et al., 2012; Prasad & Kumar, 2014; Räisänen, 2017) lead to, among other consequences, degradation and disappearance of habitats such as mobile white or grey dunes (e.g., Namura-Ochalska, 2004, 2004; Tylkowski, 2017). The greatest losses of such habitats have been observed in Southern Europe, which is connected with the development of tourism and strong urban pressure (Janssen et al., 2016). The above factors limit the habitats of psammophilous plants, animals, and fungi, which may lead to permanent extinction. Considering the modest biological variability of seashore dunes, even a minimal disturbance in their biological balance may be dangerous to the survival of species strongly dependent on this habitat. This especially refers to a small group of dune-inhabiting macrofungi, including Hohenbuehelia culmicola Bon, Laccaria maritima (Theodor.) Singer ex Huhtinen, Phallus hadriani Vent., and Psathyrella ammophila (Durieu & Lév.) P. D. Orton (Bujakiewicz & Lisiewska, 1983; Fraiture & Otto, 2015; Høiland, 2012; Watling & Rotheroe, 1989). The fungi are vulnerable because of their specific requirements of sandy nutrient-poor sites with initial vegetation (Fraiture & Otto, 2015). This type of vegetation is rare in Europe and mainly threatened by coastal management and by nitrogen deposition from air pollution (Houston, 2016; Ozinga et al., 2013).
In some European countries, P. ammophila is red-listed as an endangered species, e.g., in Austria (Dämon & Krisai-Greilhuber, 2016), Germany (Dämmrich et al., 2016), Italy (Rossi et al., 2013), Norway [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton,”2015], and Poland (Wojewoda & Ławrynowicz, 2006), while in Croatia it is under legal protection (Tkalčec & Mešić, 2008). The species is also included in The Global Fungal Red List [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton,”2020].
This paper aims to provide ecological notes, new localities for P. ammophila on the Wolin Island, and the resulting update of the distribution of this species in Poland. Moreover, the macroscopic and microscopic features of the basidiocarps of this fungus were presented.
Material and Methods
Psathyrella ammophila basidiocarps were collected from Miedzyzdroje and Świnoujście (both on Wolin Island) in August 2012 and October 2019, respectively. The descriptions of the basidiocarp morphology and ecological characteristics of this species are based on original material, accompanied by information from literature. The microscopic structures were observed and measured using a Zeiss AxioLab light microscope (LM). Dimensions of pleurocystidia, cheilocystidia, basidia (without sterigmata), and spores are based on 10–20 measurements per specimen from our collections. Size ranges of the microscopic features are given as follows: (minimum value–) first decile – ninth decile (–maximum value). Scanning electron microscope (SEM) images were captured in the Center of Molecular Biology and Biotechnology, Environmental Research Laboratory, University of Szczecin (Poland). The specimens were identified based on their macroscopic and microscopic features, using monographs by Kits van Waveren (1977, 1985), and Örstadius and Knudsen (2012).
The distribution of P. ammophila in Poland is presented on a cartogram according to the ATPOL gird square system as used by Wojewoda (2000). The cartogram is based on our investigations and all available published and unpublished data. In order to illustrate the changes in the distribution of this species, we presented the localities in two time periods: before 1970 and in 1970 or after. The fungal nomenclature and the synonyms were given according to the Index Fungorum (http://www.indexfungorum.org/) database. The names of vascular plants follow the description by Mirek et al. (2002), and Jalas and Suominen (1973), and the names of the plant communities are given according to the data in the source literature. The collected specimens of P. ammophila were deposited in the Herbarium of Szczecin University (SZUB-F), Poland.
Results
Psathyrella ammophila (Durieu & Lév.) P. D. Orton, Trans. Br. mycol. Soc. 43(2): 180 (1960) – Psathyrellaceae, Agaricales, Agaricomycetes, Agaricomycotina, Basidiomycota, Fungi
Synonymy: Agaricus ammophilus Durieu & Lév., in Durieu, Expl. Sci. Alg., Bot., Atlas 14: 31 (1846) [1846–49]; Deconica ammophila (Durieu & Lév.) Morgan, J. Mycol. 13(4): 145 (1907); Drosophila ammophila (Durieu & Lév.) Kühner & Romagn., Fl. Analyt. Champ. Supér. (Paris): 358 (1953); Psathyra ammophila (Durieu & Lév.) Quél., Bull. Soc. bot. Fr. 26: 52 (1880) [1879]; Psilocybe ammophila (Durieu & Lév.) Gillet, Hyménomycètes (Alençon): 587 (1878); for other synonymies, see Index Fungorum
Macroscopic and Microscopic Features
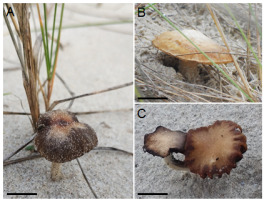
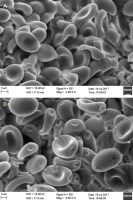
Pileus 10–40(50) mm in diam., hemispherical to broadly convex, later plane, sometimes with depressed center and deflexed margin, slightly fibrillose at first but later naked, reddish brown, dark brown to pale brown, hygrophanous, when dried ochraceous to pale brown at margin and yellowish brown at center (Figure 1); veil (white) when young; lamellae adnate, first greyish brown then dark brown with purple tinges, almost black with age; stipe 30–70 × 3–5 mm, cylindrical, hollow, whitish, light grey to pale brown, its lower part deeply sunk in the sand, sometimes with a pseudorrhiza, without a ring; spore print purplish black; taste and smell indistinctive. Pleurocystidia 39.1–68.4 × 14.8–23.4 μm, utriform, clavate, sometimes lageniform, scattered, smooth, and hyaline. Cheilocystidia of two types: (i) 24.6–63.8 × 10.2–18.8 μm similar to pleurocystidia, locally numerous, and (ii) small, clavate, numerous, especially closed to cap margin. Basidia 22.1–30.4 × 10.3–13.1 μm, clavate, with two–four sterigmata. Spores (10.7)11.4–13.7(14.8) × (5.7)6.6–7.4(8.2) μm, smooth, ellipsoid, oblong, ovoid, with suprahilar depression and large germ pore (Figure 2).
Habitat
Psathyrella ammophila is most frequently reported on seashore beaches and sand dunes, while it is considerably less common on deep inland sandy areas (e.g., on inland sand dunes). This species grows on loose or stable sands, individually or in groups of several basidiocarps, whose stems deeply penetrate the sand, frequently among shoots of a wide variety of psammophilous plants [Bujakiewicz & Lisiewska, 1983; Kits van Waveren, 1977; Kreisel, 1987; Maletti, 2014; “Psathyrella ammophila (Durieu & Lév.) P. D. Orton,”2020; Rudnicka-Jezierska, 1969; Singer, 1968]. In Europe, this fungus was most often found among shoots of Ammophila arenaria and on dead and disintegrating remains of this plant, e.g., on their leaves and roots (Courtecuisse, 1984; Dennis, 1983; Perini & Venturella, 2008). Moreover, it was also identified in the company of grasses inhabiting dunes including, among others, Agrostis stolonifera subsp. maritima (Lam.) G. Mey, Cynodon dactylon (L.) Pers., Elymus farctus (Viv.) Runemark ex Melderis, E. repens (L.) Gould, Festuca vaginata Waldst. & Kit. ex Willd., and Leymus arenarius (L.) Hochst., and herbaceous dicotyledonous plants, including Cakile maritima Scop., Euphorbia paralias L., and Salsola kali L. (Pando & Fernández, 2001; Singer, 1968). These plants form homogenous associations and pioneer communities on sand dunes, including Agropyretum mediterranei Kuhn ex Br.-Bl. 1933, Ammophiletum arundinaceae Br.-Bl. 1933 (Courtecuisse, 1984; Perini & Venturella, 2008), and Euphorbio-Ammophiletum arenariae R. Tx. 1945 (Guinberteau, 2004). In Poland, P. ammophila was found in Elymo-Ammophiletum arenariae Br.-Bl. et De Leeuw 1936, Helichryso-Jasionetum litoralis Libb. 1940, and Empetro nigri-Pinetum (Libb. et Siss. 1939 n.n.) Wojt. 1964 plant communities (Bujakiewicz & Lisiewska, 1983; Lisiewska, 1966; Rudnicka-Jezierska, 1969; Skirgiełło, 1976; Teodorowicz, 1936).
This psammophilous species was also recorded, although significantly less frequently, on more stabilized habitats such as compact turf developed on sands and dunes overgrown by trees, e.g., Pinus halepensis Mill. and P. pinea L., as well as on anthropogenic localities, e.g., on state beaches and boulevards (Pando & Fernández, 2001, and references therein).
General Distribution
Psathyrella ammophila is a widely spread species, occurring in all the continents except Antarctica, and has been reported in North America, e.g., Canada and the USA (Redhead, 1997; Smith, 1972), South America, e.g., Argentina and Uruguay (Singer, 1968; Wright & Albertó, 2002) as well as in Australia and New Zealand [“Psathyrella ammophila (Lév. & Durieu) P. D. Orton,” 2020]. In Africa, it is found, among other countries, in Algeria (Kits van Waveren, 1977; Singer, 1968) and Morocco (Malençon & Bertault, 1970), while in Asia – in China (Bi et al., 1993), Israel (Binyamini, 1994), Turkey (Kaya, 2010; Sesli & Denchev, 2008), and Pakistan (Sultana et al., 2011).
In Europe, P. ammophila occurs mainly over sandy shores of the Atlantic Ocean, the North Sea and the Baltic Sea, and the Mediterranean Sea. This species is common on the seashores of Denmark (Elborne, 1996; Örstadius & Knudsen, 2012), the Netherlands [Kits van Waveren, 1977; “Psathyrella ammophila (Durieu & Lév.) P. D. Orton,” 2020; Singer, 1968], and Italy (Maletti, 2014; Perini & Venturella, 2008, and references therein), and can often be found in Spain, including the Balearic Islands (Menéndez Valderrey, 2020; Rubio et al., 2005; Siquier & Salom, 2005; Siquier et al., 2012), and in Portugal (Pando & Fernández, 2001, and references therein). Occasionally, it is also found in Great Britain [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton,” 2020; Savage, 2008] and Ireland [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton,” 2020] as well as in the south-western Sweden (Örstadius & Knudsen, 2012; “Psathyrella ammophila,” 2015) and the south-western and central Norway (Høiland, 2006, 2012). It has been reported in scattered localities in Germany (Dämmrich et al., 2020), France (Courtecuisse, 1984; Guinberteau, 2004; Kits van Waveren, 1977), and Estonia [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton, 1960,” 2020], and the Kaliningrad District in the Russian Federation (Dedkov & Grishanov, 2010). It is also observed in Belgium [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton,” 2020], Iceland (Örstadius & Knudsen, 2012), and Latvia (Dāniele [Avota] & Krastiņa, 2002). Quite rarely, it has also been reported in other European countries including, among others, Austria (Dämon & Krisai-Greilhuber, 2016), Bulgaria (Denchev & Assyov, 2010; Denchev et al., 2007), Croatia (Tkalčec & Mešić, 2008), Hungary (“Psathyrella ammophila – Homoki porhanyósgomba,” 2020), and Slovakia (Škubla, 2003; Vašutowá, 2006).
Distribution in Poland
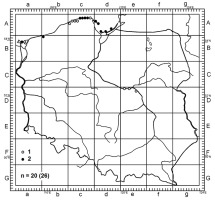
In Poland, P. ammophila has been mainly reported in the north-eastern (e.g., the Hel Peninsula, the Słowiński National Park) and western parts of the Baltic Sea coast (the Wolin National Park) (Bujakiewicz & Lisiewska, 1983; Kujawa & Gierczyk, 2013; Kujawa et al., 2020; Lisiewska, 1966; Skirgiełło, 1976; Teodorowicz, 1936). Two new localities, which were discovered after 2011, are situated on the Wolin Island. Moreover, there have been reports on the species occurrence in the central part of the country, namely the Kampinos Forest (Rudnicka-Jezierska, 1969). To date, in Poland, P. ammophila has been recorded in 26 localities, including 14 after 1970 (Figure 3).
Figure 3
Distribution of Psathyrella ammophila in Poland. 1 – localities before 1970; 2 – localities in 1970 or after; n = total number of 10 × 10-km squares (total number of localities).
New Localities
(i) Wolin Island: ca. 2.5 km W of Międzyzdroje (53°54′58.266″ N, 14°23′42.26″ E), on the white dune of the Baltic Sea coast; 2012-08-14, leg. et det. K. Mazurkiewicz-Zapałowicz, 11 basidiocarps were found growing (individually or in groups of two or three) among living shoots of Ammophila arenaria (L.) Link, near Salix sp., over an area of about 30 m2; (ii) Wolin Island: Świnoujście (53°54′58.209″ N, 14°18′19.857″ E), on the white dune of the Baltic Sea coast; 2019-10-26, leg. et det. M. Stasińska, about 40 basidiocarps were reported, growing (individually or in groups of two to four) mainly among living and dead shoots of A. arenaria, and near Artemisia campestris L., along a distance of 250 m.
List of Localities of P. ammophila in Poland2
Ac-34 – Stilo-Osetnik (Wantoch-Rekowski, 2014; unpubl.). Ac-35 – Lubiatowo, 2 km N (Domian, 2016; unpubl.). Ac-36 – Białogóra (Kujawa & Gierczyk, 2013). Ac-37 – (i) Dębki (Teodorowicz, 1936); (ii) Widowo res. (Kujawa & Gierczyk, 2013). Ac-38 – Karwia (Teodorowicz, 1936). Ac-41 – Czołpino (Bujakiewicz & Lisiewska, 1983). Ac-42 – Mierzeja Łebska (Bujakiewicz & Lisiewska, 1983). Ac-43 – Łeba (Dominik, 1951). Ac-50 – Rowy (Bujakiewicz & Lisiewska, 1983). Ad-40 – Kuźnica (Soboń, 2008; unpubl.). Ad-51 – (i) bet. Jurata and Hel (Tylińska, 2014; unpubl.); (ii) Jurata (Wantoch-Rekowski, 2014; unpubl.). Ad-76 – Piaski (Neubauer & Neubauer, 2013; unpubl.). Ad-82 – (i) Gdańsk-Orlinki (Neubauer & Neubauer, 2012, 2013; unpubl.); (ii) Mikoszewo (Wantoch-Rekowski, 2014; unpubl.). Ad-84 – bet. Sztutowo and Kąty Rybackie (Neubauer & Neubauer, 2014; unpubl.). Ba-22 – (i) Międzyzdroje, 2.5 km W (Mazurkiewicz-Zapałowicz, 2012, SZUB-F 2227; unpubl.); (ii) Świnoujście (Stasińska, 2019, SZUB-F 2226; unpubl.). Ba-23 – (i) bet. Grodno and Wisełka (Lisiewska, 1966); (ii) around Wisełka (Lisiewska, 1966); (iii) Gosań Mt (Lisiewska, 1966). Bb-00 – Kołobrzeg, E part of the city (Twardy, 2013; unpubl.). De-01 – Kamion, Kampinos Forest (Rudnicka-Jezierska, 1969). De-03 – Grochale, Kampinos Forest (Rudnicka-Jezierska, 1969). De-15 – Truskaw, Kampinos Forest (Rudnicka-Jezierska, 1969).
Discussion and Conclusions
Although P. ammophila is a widely spread fungus, mainly in Europe (Dämmrich et al., 2020; Dämon & Krisai-Greilhuber, 2016; Maletti, 2014; Örstadius & Knudsen, 2012; Perini & Venturella, 2008), the current knowledge on its geographic distribution is still incomplete and requires verification. For instance, the information found in the literature on the occurrence of this fungus in the Czech Republic is incorrect (Vašutowá, 2006). In Poland, after 1970, 14 localities of this species were reported, including 13 localities identified in the last 10 years, which constitute 50% of all 26 localities recorded to date. The occurrence of P. ammophila in the Kampinos Forest, where it was observed in the 1960s by Rudnicka-Jezierska (1969), was not confirmed (Karasiński et al., 2015). In the last few years, this fungus has mainly been noted in the north-eastern part of the Polish seashore, e.g., the Hel Peninsula (Kujawa & Gierczyk, 2013; Kujawa et al., 2020), where it was found in the 1930s and reported by Teodorowicz (1936) as a very common species. Two new localities are situated further West, on Wolin Island. They neighbor a few other localities of the north-eastern seashores of Germany, including the Uznam and Rugia islands (Dämmrich et al., 2020).
The typical habitat of P. ammophila includes mobile white sand dunes, which occur along seashores and are classified as Natura 2000 habitats (Janssen et al., 2016). This habitat is subjected to strong man-made-disturbances due to the escalating development of tourism in the last 100 years, which has led to a reduction of its areas. It can be forecast that the pressure from tourism will grow and fungi habitats will further degrade. Apart from anthropogenic factors, natural factors such as abrasion of seashores and vegetation succession also have a negative effect on the habitats of the fungus (Jakusik et al., 2012; Janssen et al., 2016; Leuschner & Ellenberg, 2017; Tylkowski, 2017). According to Leuschner and Ellenberg (2017), a major part of the German and Polish shore of the Baltic Sea has been eroded, causing the disappearance of sand dunes, which are otherwise colonized and stabilized by plants and fungi, including P. ammophila (Høiland, 2012; Watling & Rotheroe, 1989).
The destruction of seashore sand dunes, observed for many years, has made P. ammophila an endangered taxon, although the level of this negative effect depends on the region. In Croatia (Tkalčec & Mešić, 2008) and Austria (Dämon & Krisai-Greilhuber, 2016) this fungus is classified as “critically endangered” (CR). Moreover, in the latter country, this fungus has not been found in the recent 10 years and this may lead to its inclusion into a higher threat category. Such amendment has already been done in Norway, where over a dozen years, the category was changed from “near threatened” (NT) (Kålås et al., 2006) to “vulnerable” (VU) (Brandrud et al., 2010), and then to “endangered” (EN) [“Psathyrella ammophila (Durieu & Lév.) P. D. Orton,” 2015). In Slovakia, the species is classified as “endangered” (EN) (Lizon, 2001), while in Sweden (“Psathyrella ammophila,” 2015) and Italy (Rossi et al., 2013) – “near threatened” (NT). In Hungary, P. ammophila is listed in category 3 (“care-demanding species”) and received the lowest score on a three-score scale (Siller & Vasas, 1995). On the German red list of fungi, the species was assigned to category G (“endangerment of unknown”) (Dämmrich et al., 2016). However, in some of the German federal states, P. ammophila is classified in different categories: in Baden-Wurttemberg (Winterhoff & Krieglsteiner, 1984) and Rhineland-Palatinate (Zehfuß et al., 1999) it is in category 1, while in Mecklenburg-Vorpommerns (Schwik, 1999) and Schleswig-Holsteins (Lüderitz, 2001) in category 3. In Poland, P. ammophila was in 2006 classified as an “endangered” (E) taxon (Wojewoda & Ławrynowicz, 2006).
Psathyrella ammophila is a very important fungal element of the Baltic seashore dunes, as only a few species are capable of colonizing mobile dunes. Due to the progressive degradation of these ecosystems and climatic changes, the perspective for the next several years seems unfavorable to P. ammophila. Considering all the above facts, this fungus should be listed as a protected species.
Handling Editor
Małgorzata Ruszkiewicz-Michalska; Institute for Agricultural and Forest Environment, Polish Academy of Sciences, Poland; University of Łódź, Poland; https://orcid.org/0000-0001-8901-0552