Introduction
The introduction of some North American tree species into Europe was initiated by Lord Weymouth in 1605 (Pinus strobus L.; white pine), followed by David Douglas in 1827 [Pseudotsuga menziesii (Mirb.) Franco; Douglas fir] and John Jeffrey in 1853 (Thuja plicata D. Don.; red cedar). However, the large-scale introduction of nonnative tree species dates back to the end of the nineteenth century (Białobok & Chylarecki, 1965; Chylarecki, 1976; Jaworski, 2011), mainly owing to the need to obtain a faster-growing wood stock (Zobel et al., 1987) with high biocenotic values in forest cultivation (Bellon et al., 1977; Gazda & Fijała, 2010; Gazda & Szlaga, 2008; Herman & Lavender, 1999; Szwagrzyk, 2000).
In Poland, nonnative trees were introduced in the Kórnik Arboretum (1861), Wirty Arboretum (1881), and in the north-eastern areas of Poland within the present borders (Warmia and Mazury) (1861 and 1880) (Białobok & Chylarecki, 1965; Cyzman et al., 2012; Panka, 2012; Schwappach, 1891; Szymanowski, 1959; Tumiłowicz, 1967, 1968, 1988). The existing groups (clusters) have been the subject of numerous ecological and forestry studies (Bellon et al., 1977; Tumiłowicz, 1967), including few phytopathological/mycological experiments (Benben, 1969; Grzywacz, 1978, 1979; Grzywacz et al., 1998). Such groups of trees (the forest area of the Regional Directorate of State Forests in Olsztyn) are currently 100–135 years old. In the meantime, stands have been subjected to various environmental factors such as industrial emissions or weather anomalies; they have also been colonized by various insects or fungi (personal comment from forest districts).
For cognitive and practical (economic) reasons, it is essential to state the native species of macromycetes (mycorrhizal and wood-inhabiting) that may colonize the nonnative trees and affect their development and survival. The question about the “strangeness” of these species in the boreal forests of Europe, including Poland, remains open and needs to be further investigated. In this context, we hypothesized that the species composition of macromycetes inhabiting trees of three different species introduced into Warmia and Mazury in the nineteenth century does not differ from those recorded on native tree species in this region. The question is to what extent the identified species may threaten the future existence of nonnative trees.
Material and Methods
Study Area
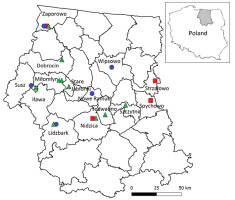
The study was performed in old areas of the former Prussian Experimental Station (PES) research plots, where 23 tree species native to North America were introduced and cultivated in the 1890s (Danckelmann, 1884; Schwappach, 1891, 1901, 1911). The shape and size of the plots previously laid (2–12 circular areas or quadrilateral areas of a similar size) can be associated with the commonly used Mortzfeld nesting complete cutting (Mortzfeld, 1896). In 1962–1963, Tumiłowicz (1967) assessed the trees remaining in the plots in the Masurian-Podlasie region. Three species exhibited the highest survival rates, namely Douglas fir (Pseudotsuga menziesii), white pine (Pinus strobus), and red cedar (Thuja plicata) (Figure 1). Among the locations created by PES, the following forest districts need to be mentioned: Dobrocin, Jedwabno, Lidzbark, Miłomłyn, Nidzica, Nowe Ramuki, Spychowo, Strzałowo, Susz, Szczytno, Wipsowo, and Zaporowo (Figure 2).
Plots Tested
In 2016, based on an available forest database (https://www.bdl.lasy.gov.pl/), stands with introduced species were GPS-located in 44 areas (Figure 2); however, only 19 cases were confirmed, where clusters founded by Danckelmann (1884) and Schwappach (Schwappach, 1891, 1901, 1911) and inventoried by Tumiłowicz (1967, 1968, 1988) were found (in other cases, the number of trees was too small to be assessed) (Table 1).
In these plots (Table 1), the trees were measured, and the fungi occurring (i) on the trees and (ii) accidentally present during the observation in their immediate vicinity were inventoried from August to November (2018–2019). The forest floor in a radius of 0.5 m from the tree trunk was adopted as the minimal area (Moravec, 1973). During the assessment, all sporocarps present on tree trunks, root collars, and stumps as well as the minimal area of the investigated tree species were identified and counted (Figure 3). The assessed plot was similar, depending on the diameter of the tree in the root collar for white pine (1.85 m2 in diameter), Douglas fir (1.6 m2), and red cedar (1.71 m2).
In all investigated plots (Table 1), all standing trees were assessed, and dendrometrical parameters were determined (detailed data not published). For every Forest District from 30 days before assessment, the average air temperature and sum of rainfall as well as hydrothermal coefficient K (Sielianinov’s index applied to assess thermal and pluviometric conditions in agronomy) were determined (
Table 1
Characteristics of plots with introduced tree species in the Forest Districts and average monthly temperature and rainfall and values of hydrothermal coefficient K.
The collected material was investigated using standard methods applied to the taxonomy of macromycetes. Macromycetes were identified both on site and in the laboratory. Species were identified using keys (Bernicchia, 2005; Bernicchia & Gorjón, 2010; Breitenbach & Kränzlin, 1984, 1986, 1991, 1995, 2000; Kränzlin, 2005; Ludwig, 2007; Ryvarden & Melo, 2017). No genetic analyses were performed. Most specimens were deposited at the Department of Entomology, Phytopathology and Molecular Diagnostics at the University of Warmia and Mazury, Olsztyn, Poland.
The results were compiled for tree species and place, without division into forest districts and departments. The fungal nomenclature follows the Index Fungorum database (http://www.indexfungorum.org/). Threat categories were assigned according to the “Red list of the macrofungi in Poland” (Wojewoda & Ławrynowicz, 2006). The names of trees are cited according to Mirek et al. (2002).
The frequency of occurrence of fungi on the litter layer was determined according to the following scale: + individually (one–two sporocarps), ++ rarely (3–10), +++ often (>10). Ecological indices (Weiner, 2012) describing the diversity and comparing the species composition of macromycetes recorded on the wood (Gómez-Hernández & Williams-Linera, 2011; Kujawa & Kujawa, 2008; Piętka et al., 2019) were determined on all plots (Table 2).
Table 2
Ecological indices used in the assessment.
Results
The species richness of fungi directly related to P. menziesii (36 taxa in total) was considerably higher than that for fungi related to P. strobus (19 taxa) and T. plicata (10 taxa). The beneficial effect of the vicinity of a tree trunk was particularly pronounced for P. menziesii because 17 taxa (including 10 ectomycorrhizal taxa) of fungi were found in the litter and soil around this species. In contrast, only four taxa (including two ectomycorrhizal taxa) were recorded around P. strobus and T. plicata (Table 3, Table 4).
Table 3
General description of the studied plots containing introduced tree species.
In total, 48 fungal taxa were identified in the three nonnative tree species, of which, 46 belonged to Basidiomycota and two to Ascomycota (Table 4).
The richness of taxa inhabiting wood or bark and stumps of assessed trees was an indicator of the possible coexistence of particular macromycetes with assessed trees. We identified 19 taxa with P. menziesii wood, 15 with P. strobus wood, and six with T. plicata wood (Table 4). The most common fungi were typical saprotrophs (e.g., Stereum sanguinolentum, Trametes versicolor, Hypholoma fasciculare) as well as Basidiomycota, which are pathogenic to conifers, such as Heterobasidion sp. (40 basidiocarps), Armillaria sp. (26), Phaeolus schweinitzii (11), and Porodaedalea pini (six). Of these, Armillaria sp. and Heterobasidion sp. were recorded only on P. strobus and T. plicata; however, Heterobasidion sp. basidiocarps or specific wood decay symptoms were found mainly on the root collar and stumps of P. strobus (Figure 4). The highest number of basidiocarps (91) on the wood of one tree species was Trichaptum fuscoviolaceum on P. strobus (Table 4).
Table 4
Taxa of fungi on the litter layer within a radius of 0.5 m around the trunk and number of sporocarps on trunks and stumps of assessed trees on all plots.
Figure 4
Heterobasidion annosum basidiocarpson the T. plicata root collar (left), Hypholoma fasciculare on P. strobus stump (middle), and Phaeolus schwinitzii on root of Pseudotsuga menziesii.

Indices of species diversity and dominance indicate similar species richness of the observed taxa on the plots of P. strobus and P. menziesii (Table 5). The Shannon, Simpson, and Pielou index values exhibited a slightly greater community diversity on the P. strobus trees. In contrast, the Margalef’s index values indicated the greatest community diversity in the P. menziesii trees, which was the result of a more favorable ratio of the number of sporocarps on wood to the number of identified fungal species. The dominance index was slightly higher for P. menziesii trees (0.16) than for P. strobus trees (0.12), whereas T. plicata trees were characterized by the smallest species richness and a high Simpson’s dominance rate (0.28). The obtained effect was the result of a relatively small number of sporocarps, which mainly belonged to Heterobasidion annosum.
Table 5
Ecological index values for Pseudotsuga menziesii, Pinus strobus, and Thuja plicata trees in the assessed sites.
Table 6
Sørensen’s index of similarity among the tree species in the assessed sites.
Variant | Sørensen’s index (%) |
Pinus strobus vs. Pseudotsuga menziesii | 47.1 |
Thuja plicata vs. Pseudotsuga menziesii | 16.0 |
Thuja plicata vs. Pinus strobus | 47.6 |
The species composition of fungi inhabiting wood of T. plicata and P. menziesii coincided only in 16% similarties. In P. strobus clusters, the species composition of macromycetes was approximately 47% identical to that of T. plicata and P. menziesii (Table 6).
Discussion
The number of macromycetes species whose sporocarps were located around and on the wood of the investigated nonnative trees was relatively high. When assessing the frequency of sporocarps near trees, it was assumed that water flow through branches and trunks during rainfall (in terms of its additional chemical or microbiological composition) could have influenced both the soil and vegetation around the tree (Bollen et al., 1968; Dunkerley, 2020; Gersper & Holowaychuck, 1971). Such influences could also interact with the ectomycorrhizal mycelia and sporocarps (Lehto & Zwiazek, 2011). We detected 48 macromycetes, of which 21 taxa were found on the litter layer and soil within the minimal area surrounding the trees, and 27 inhabited the wood of standing and lying trees and tree stumps. The number of species found on the litter layer and the soil as well as the sporocarps on trees and stumps differed among not only the three tree species but also forest districts (not compared here). These individual observations must be repeated in additional studies.
The sporocarps inhabiting P. menziesii and P. strobus trees represented 19 and 15 taxa, respectively, whereas on T. plicata, only six species were found. In the vicinity of P. menziesii, P. strobus, and T. plicata, 17, four, and four taxa were found, respectively. Smith et al. (2002), assessing the abundance of sporocarps of fungi in old P. menziesii stands in Oregon, described 14 taxa which they represented the genera Cantharellus, Cortinarius, Lactarius, Russula, and Thelephora among them, which were also found in the present study.
The species richness of fungi both around the trees and on the trees varied, depending on the host species. We mostly observed single species with different numbers of sporocarps; however, among the identified species, Fomitopsis pinicola and Leptoporus mollis were found on the wood of all three tree species or, in some cases, only on two species, but always on P. strobus.
The five species collected were red-listed fungi. One of them (L. mollis) has been listed in the highest threat category, described as endangered (E), whereas four (Calocera furcata, Physisporinus vitreus, Porodaedalea pini, and Serpula himantioides) are rare (R) (Wojewoda & Ławrynowicz, 2006). Among the identified species, nine (Anthrodia xantha, Calocera furcata, Exidia pithya, E. saccharina, L. mollis, Phaeolus schweinitzii, Physisporinus vitreus, Porodaedalea pini, and Serpula himantioides) have been listed in the “Register of protected and endangered fungal species in Poland (GREJ)” (Kujawa et al., 2020). Butin (1995) indicated a low harmfulness of P. pini on Pseudotsuga; however, the basidiocarps were not found on the studied P. menziesii trees, whereas it was sporadically reported on P. strobus.
The number of sporocarps found around the trees in all plots was assessed once and could not be treated as indicative of the richness of the population. One should also take into account the different temperature and rainfall conditions during the assessment period and in the preceding months, which affect fungal fruiting. Field notes indicate that there were both individual specimens (e.g., Phallus impudicus) and groups of sporocarps (e.g., Mycena spp.), most frequently found on plots with P. menziesii. When comparing the epigeous sporocarps list with the mycorrhizal species found in some the habitats occupied by Scots pine (Rudawska et al., 2011; Rudawska et al., 2018), a small scale of similarity should be noted; e.g., in the case of Cortinarius sp., Lactarius spp., Imleria sp., or Russula sp. Kwaśna et al. (2019) found in the soil on Scots pine site some other genus present in areas described here, e.g.: Armillaria sp., Cantharellus sp., Lactarius sp., and Thelephora sp.
The number of sporocarps on trees and stumps in all plots ranged from one (Serpula himantioides, Pholiota sp.) to about 90 (e.g., Trichaptum fuscoviolaceum, Trametes versicolor, Stereum sanguinolentum). Considering the quantitative data for the minimal area and the number of trees assessed, it can be assumed that the macromycete turnout was comparable to the results obtained for P. menziesii in the other localities (Smith et al., 2002) as well as to richness of fungi inhabiting the wood of 120-year-old Scots pine (Pinus sylvestris L.) trees in the “Sosna Taborska” site (Miłomłyn Forest District), both in the reserve stand and in the managed forest. Piętka et al. (2019) described a total of 32 taxa, with 25 being in the reserve stand and 12 in the managed stand. Kwaśna et al. (2017) recorded wood samples of Scots pine basidiomata of four taxa (Armillaria ostoyae, Heterobasidion annosum, Hebeloma fasciculare, and Trichaptum fuscoviolaceum), which were also identified in the present study. Sierota et al. (2016) found Phlebia tremellosa (identified here) on Norway spruce stumps; however, Phlebiopsis gigantea, a common fungus on coniferous stumps and in roots in Poland (Sierota, 1995), was not recorded on stumps of three assessed trees. Pathogen Heterobasidion annosum is often noted on P. strobus, and T. plicata is described as a frequent partner of Scots pine and Norway spruce in Poland (Cieślak et al., 2011).
However, comparison of our results with previous studies is difficult owing to the lack of similar research in Poland. Nonetheless, Grzywacz (1978) and Grzywacz et al. (1998), during a study conducted in 1973–1998, found 91 fungal taxa associated with Douglas fir, including approximately 40 taxa classified as macromycetes. Our research expanded the list of macrofungi occurring on P. mensiesii in the Polish forests by another 14 taxa (Antrodia xantha, Ascocoryne sp., Calocera furcata, Clavaria incarnata, Cylindrobasidium laeve, Ganoderma applanatum, L. mollis, Mycena viridimarginata, Phlebia radiata, P. tremellosa, Physisoporinus vitreus, Skeletocutis amorpha, Tapinella atrotomentosa, and Xylaria hypoxylon).
In the studies cited above (Grzywacz, 1978; Grzywacz et al., 1998), the number of taxa found on P. strobus was 64 (including about 20 macrofungi), whereas on T. plicata, 59 taxa were found (including 13 macrofungi). In this study, we identified 10 taxa on P. strobus that have not yet been reported in association with P. strobus for Poland (Cylindrobasidium laeve, Dacrymyces sp., Exidia pithya, E. saccharina, Gymnopilus penetrans, Leptoporus mollis, Mycena sanguinolenta, Tapinella panuoides, Trametes versicolor, and Xylaria hypoxylon) and three taxa on Thuja plicata (Exidia pithya, Leptoporus mollis, and Serpula himantioides).
In the present study, macromycetes were not distinguished separately for standing, lying trees, or stumps. However, it was found that on living trees of P. menziesii, they were recorded sporadically, as reported by Michel et al. (2011), and usually in the root collar of trees. However, they were most often found on snags, logs, and few stumps. The volume of dead wood, an ecological niche for fungi and other organisms, differs from 7.1 m3/ha for P. menziesii clusters to 56.5 m3/ha for T. plicata ones. This accounted for 1.4%–4.9% of tree biomass in these clusters, which corresponds to an average of 2–5 m3/ha in State Forests in Poland (Skwarek & Bijak, 2015).
Macromycetes inhabiting the evaluated nonnative trees (wood, bark, roots, snags, stumps, litter, and soil 0.5 m around the trunk) were characteristic of fungi reported on native conifers in Poland. The highest richness of fungal species was recorded for Douglas fir and white pine and the lowest for red cedar. Among the fungi found, the presence of root pathogens from the genera Armillaria and Heterobasidion was recorded, albeit only on P. strobus and T. plicata trees. Pseudotsuga menziesii trees showed the most robust health (evaluated on the basis of colonization by fungi and deadwood) among the nonnative trees assessed in all locations.
Handling Editor
Anna Kujawa; Institute for Agricultural and Forest Environment, Polish Academy of Sciences, Poland; https://orcid.org/0000-0001-9346-2674