. Introduction
Currently, a universal need exists worldwide to guarantee food security and the production of raw materials for agriculture. In addition, efficient and inexpensive agricultural techniques to increase the production of food and cash crops must be developed to meet the needs of the agricultural, pharmaceutical, and environmental sectors. However, agricultural production has been severely reduced due to the environmental consequences of anthropogenic activities. Despite this reduction, agricultural production has also become an increasingly important source of greenhouse gases while simultaneously providing a means to mitigate climate change through carbon storage in soils and vegetation (FAO, 2019). Regardless of the potential benefits, agricultural production has had profound effects on the environment.
The excessive use of agrochemicals in modern agriculture has led to numerous undesirable effects, including immediate contamination of the abiotic environment (soil, groundwater, and air) and adverse impacts on beneficial organisms, such as natural pest predators (Mortensen et al., 2000; Peterson et al., 2018). Furthermore, agrochemicals can cause physiological harm to non-target organisms, including humans, and contribute to long-term environmental degradation (WHO, 2022). Given these significant challenges, there is an urgent need for sustainable alternatives that can mitigate these effects while maintaining high agricultural productivity, such as developing and applying plant biostimulants. According to the European Biostimulant Industry Council (EBIC) (EBIC, 2019), “plant biostimulants are materials that contain substances and/or microorganisms whose function, when applied to plants or the rhizosphere, is to stimulate natural processes that improve or support nutrient absorption, nutrient efficiency, tolerance to abiotic stress, and crop quality, regardless of their content.” Yakhin et al. (2017) added that “a biostimulant is a formulated product of biological origin that improves plant productivity as a consequence of the novel or emerging properties of the complex of constituents and not as a sole consequence of the presence of known essential plant nutrients, plant growth regulators, or plant protective compounds.
A sustainable alternative to using natural resources is to obtain biostimulants from botanical extracts. An existing idea among researchers is that the activity of any extract should not depend on its nutritional value but rather on the origin of its bioactive ingredients (i.e., chemical composition) (Luke et al., 2021).
Verbesina sphaerocephala A. Gray, commonly known as capitaneja or white rod, is a herbaceous plant of the Asteraceae family and is endemic to western Mexico (Jalisco, Michoacán, Nayarit, Guanajuato, and Guerrero) (Rzedowski et al., 2011). Due to its wide distribution and propensity to occupy disturbed environments, V. sphaerocephala is not threatened. As such, V. sphaerocephala has been proposed as a potential source of botanical extracts and biostimulants that may be combined to increase crop yields (Rodríguez-Valdovinos et al., 2021). In particular, the agricultural potential of V. sphaerocephala as a plant growth promoter has already been reported in the development of American cucumber (Cucumis sativus) and Fragaria × ananassa (Hernández-Pérez, 2020; Velasco-Ramírez et al., 2022a). Therefore, the application of V. sphaerocephala extracts may also support the sustainable production of important agricultural crops, such as tomato (Solanum lycopersicum), which is currently the most produced vegetable worldwide (117,118,248 t y-1) (SIAP, 2020).
Mexico is the ninth most important producer of tomato in the world (3,324,263 t per year) (SIAP, 2022). The main problem facing tomato production in Mexico is the intensive application of chemical fertilizers that damages soils and agricultural systems that depend on them (Hernández et al., 2013). Thus, there is an urgent need to develop biostimulant preparations with a wide spectrum of functional properties that may be combined with other beneficial agents and applied to multiple crops. Therefore, the objective of this study was to analyze the components of the botanical extracts of V. sphaerocephala and the type of extract application (foliar, substrate, and foliar + substrate) on the growth and yield of tomato plants (S. lycopersicum) in greenhouse conditions.
. Materials and methods
Study Site
This study was developed at the University Center for Biological and Agricultural Sciences. We worked in a greenhouse (~1650 m. a.s.l.) of the Division of Agronomic Sciences of the University of Guadalajara, located in Zapopan, Jalisco, Mexico (20° 45' N and 103° 31' W).
Preparation of the botanical extract of Verbesina sphaerocephala
Healthy (asymptomatic) young leaves (vegetative phase) of wild V. sphaerocephala were collected from the hills surrounding the community of San Martin de las Flores in the municipality of San Pedro Tlaquepaque, Jalisco (20.585278° N, 103.282778° W; 1540 m. a.s.l.). The leaves were dried at room temperature in the laboratory (~26 °C) and then pulverized in an 80,335-blade mill (Hamilton Beach, Glen Allen, USA). Subsequently, we weighed 2.5, 7.0, and 1.5 g of tissue for every liter of water. We prepared the aqueous extract as an infusion. The plant powder was added after heating the water to 85 °C, and the mixture was kept under constant stirring for 10 min. The hot extracts were passed through Whatman No. 40 filter paper (Maidstone, UK) and stored in glass jars at 4 °C. The liquid extracts of V. sphaerocephala were designated as mother solutions, and their pH and EC (dS m−1) were measured.
Chemical characterization of the V. sphaerocephala aqueous extracts
For the characterization of the aqueous extracts of V. sphaerocephala, a multiparametric photometer (HI83325, Hanna Instruments, Woonsocket, USA) was used to measure pH and electrical conductivity (EC). The mineral concentration of ammonium nitrate (NH4NO3), Ca, Mg, nitrate, phosphate (PO43-), and K were determined following the procedures described by Velasco-Ramírez et al. (2022b). The chemical parameters were determined in triplicate.
In the first instance, qualitative analysis was performed to identify the possible presence of saponins, flavonoids, tannins, phenolic compounds, anthocyanins, quinones, alkaloids, reducing sugars, and anthraquinone heterosides (Peña et al., 2023); then we proceeded to realize a quantitative analysis to identify alkaloids, total phenols, total flavones and flavonols, total phenolic acids, total proanthocyanidins, total anthocyanins, and total tannins (hydrolyzable and condensed).
The quantitative determinations were analyzed spectrophotometrically. The alkaloid analysis was based on a precipitation reaction using the method proposed by Sreevidya & Mehorotra (2003) with some modifications. The contents of alkaloids are expressed as mg Alc g-1 DM (mg alkaloids per gram of dry matter). The determination of the total phenol content was based on a colorimetric oxidation-reduction reaction according to the methods proposed by Curifuta et al. (2012); the results are expressed as mg EAG/g dry material (mg gallic acid equivalents per gram of dry matter). Total phenolic acids were determined according to the methodology developed by Matkowski et al. (2008) with some modifications. The method was based on the reaction of hydroxycinnamic acids in an acidic medium with Arnow’s reagent. The results are expressed as mg EAC/g dry material (mg caffeic acid equivalents per gram of dry matter). Total flavones and flavonols were determined based on the formation of a colored complex between Al (III) ions and the carbonyl and hydroxyl groups of the flavonoid, as described by Cimpoiu et al. (2011). The contents of total flavones and flavonols are expressed as mg EQ/g dry material (mg quercetin equivalents per gram of dry matter). The analysis of total proanthocyanidins followed the method proposed by Price et al. (1978) with some modifications, which consisted of the degree of polymerization of proanthocyanidin in acidic medium; the total proanthocyanidin content is expressed as mg ECT g-1 DM (mg catechin equivalents per gram of dry matter). Total anthocyanins were evaluated via pH differences according to the method proposed by Martínez-Cruz et al. (2011). The total anthocyanins are expressed as mg EC3G/g of MS (mg cyanidin equivalents 3-glucoside per gram of dry matter). Total tannins (hydrolyzable and condensed) were obtained following the methodology used by Ricco et al. (2011) with some modifications. It was determined through the precipitation of tannins with bovine serum albumin fraction V SAB. The results are expressed as mg EAG/g dry material (mg gallic acid equivalents per gram of dry matter).
Fourier-Transformed infrared (FT-IR) spectroscopy
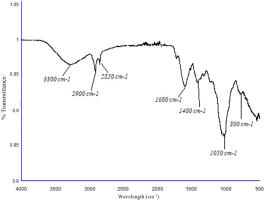
The secondary metabolites in V. sphaerocephala were investigated using FT-IR spectroscopy (Thermo Scientific, model NICOLET iS50FT-IR) on dried crude extracts. The FT-IR analysis technique utilizes infrared radiation to investigate the test samples and determine their chemical characteristics. The sample’s constituents presented light absorption within the infrared range of the electromagnetic spectrum, which is accurately relationated with the bonds found in the sample (Sobuj et al., 2024).
Growth of tomato plants and establishment inside the greenhouse
Certified seeds of S. lycopersicum (indeterminate variety PaiPai) were acquired from Enza Zaden (Enkhuizen, The Netherlands). This variety for protected culture offers continuous fruit setting. It is easily adaptable to non-extreme hot or cold weather conditions. It ripens well and has great firmness and an intense red shiny color. It is a very productive plant for early cultivation. In September 2022, 450 S. lycopersicum seeds were sown in 200 cavity polystyrene trays using a substrate composed of 50% peat moss and 50% vermiculite. The seeds were sown in greenhouse conditions (~25 °C and 75% RH) and irrigated with well water (pH 7.0; electrical conductivity [EC] of 0.15 mS/cm). The seeds were irrigated every 4 h with enough water to leave the substrate at field capacity (5 irrigations per day). Hydroponic nutrition (25% Steiner solution) was provided every 3 d until the substrate reached field capacity.
The plants were transplanted 45 days after sowing. We transplanted the plants in greenhouse conditions (double tunnel with 70/30 opacity plastic roof) into slabs (Astur-Pro Construction Ltd., UK) measuring 100 × 15 × 7.5 cm with substrate composed of 70% coconut coir and 30% coconut dust. The slabs were equipped with drip irrigation systems (2-L H-1 drippers). Each slab held 3 plants. Hydroponic nutrition was provided according to the methods proposed by Velasco-Hernández et al. (2011).
The pH and EC of the nutrient solution were 5.40 and 3.15 mS/cm, respectively. The plants were irrigated with 700 mL day-1 in the vegetative stage. Once flowering and fruit set were observed, irrigation was increased to 1.5 L day-1. The crops were maintained with 2 stems per plant, eliminating the axillary shoots that appeared every 3 days and the oldest leaves every 22 days.
Throughout the experiment, the following management activities were conducted: thinning, elimination of premature fruits, elimination of undergrowth, and implementation of ties as tutors (Figure 1).
Experimental design, extract application, and agronomic variables
A total of 10 different treatments were established. The first treatment served as a control in which plants were grown without the application of a botanical extract of V. sphaerocephala. Two factors were randomized for the other 9 treatments, in which the botanical extracts were applied. The first factor was the method of application of the botanical extracts (foliar spray, soil drench, or a combination of both). The second factor was the control treatment and the concentration of the botanical extracts (0.25, 0.75, and 1.5 g/L). The experimental units were arranged in a completely randomized two-factorial design and received either 345 mL of well water (control), 300 mL of extract applied to the substrate, or 45 mL of extract per plant applied via microdroplet spraying with hollow cone plastic nozzles.
The experimental design was completely randomized (10 treatments with 10 repetitions (3 plants per repetition)). The extracts were applied on 25 DAT and subsequently applied every 7 days until the end of the experiment. The growth characteristics (plant height of only the main stem measured with a flexometer), the leaf number (sum of both stems), and the reproductive characteristics (floral structures (sum of both stems)) were assessed. Days until flowering were measured in chronological order by selecting fully formed floral buttons about to open until senescence, and the number of fruits (sum of both stems) from fruit development to harvest time was determined. For the calculation of the yield per plant, only fruits that were in physiological maturity on the 90th DAT were harvested and counted. The number of fruits per plant was counted, and each fruit was weighed individually on a digital scale (Highland®). The sum of the individual weights of each fruit represented the performance per plant (kg plant-1).
Data were collected on 60, 75, and 90 DAT of all the variables.
Data analysis
A two-way analysis of variance was conducted (p ≤ 0.05) with the application method as factor A (substrate, foliage, or substrate + foliage), and factor B was the extract concentration (0.25, 0.75, and 1.5%). When significant differences were present, they were evaluated with the least significant difference (LSD) multiple comparison test (p ≤ 0.05). All analyses were conducted in Statgraphics® Centurion XV for Windows. To identify patterns among the chemical compositions of the botanical extracts, joint principal component analysis (PCA) and cluster analysis were performed on the normalized data (i.e., pH and EC), and the secondary metabolites were determined at each of the concentrations of the botanical extract were determined. A second PCA and cluster analysis were performed to establish whether any relationships existed between the physicochemical composition of the botanical extracts, the application method, and any growth benefits observed in the tomato plants. The cluster groups, PCA plots, correlation values, and statistical analyses were generated using Statgraphics® Centurion XVI. II. (StatPoint Technologies, Inc. The Plains, USA).
. Results
Physicochemical analysis of the botanical extract of V. sphaerocephala
The V. sphaerocephala extract, which had an intense green color, was alkaline (pH 8.33), and its EC value was 1.3 dS m-1; its mineral concentrations are shown in Table 1.
Table 1
Chemical properties of V. sphaerocephala extracts.
| Parameter | Value |
|---|---|
| pH | 8.33 |
| EC (dS m-1) | 1.31 |
| Color | Green |
| Mineral (mg/L) | Value |
| Ammonia (NH4NO3) | 0.3 |
| Calcium | 48 |
| Magnesium | 87 |
| Nitrate | Nd |
| Phosphate | 11 |
| Potassium | Nd |
The results of the qualitative tests of the botanical extracts confirmed the presence of different secondary metabolites (Table 2); among the results obtained with the aqueous extract, flavonoids, tannins, quinines, and reducing sugars stood out.
Table 2
Qualitative phytochemical analysis of Verbesina sphaerocephala aqueous extracts.
The results obtained in the aqueous extract of Verbesina sphaerocephala (Table 3) showed that the metabolites with the greatest concentration were total phenols, total flavones and flavanols, and total proanthocyanins (239.39±31.25 – 234.25±83.11, 241.27±50.47 – 235.33±58.44, 386.96±92.54 – 376.89±69.90, respectively).
Table 3
Secondary metabolites present in the aqueous extracts of Verbesina sphaerocephala.
[i] V.s (Verbesina sphaerocephala); mg EAG/g DM (mg gallic acid equivalents per gram of dry matter); mg EAC/g DM (mg caffeic acid equivalents per gram of dry matter); mg EQ/g DM (mg quercetin equivalents per gram of dry matter); mg ECT/g DM (mg catechin equivalents per gram of dry matter); mg EC3G/g of DM (mg cyanidin-3-glucoside equivalents per gram of dry matter).
FT-IR spectrum of Verbesina sphaerocephala
In the subsequent spectrum (Figure 2), the peaks observed at 3300 cm-1 indicated the presence of amines in the plant extract, and the signals obtained at 2900 and 2850 cm-1 showed the presence of aliphatic hydrocarbons, specifically alkenes. The peak distinguished at 1600 cm-1 is characteristic of the C = C - NH2 bond; subsequently, a representative signal was observed at 1400 cm-1, which confirms the existence of the C = C double bond. An important signal at 1050 cm-1 typical of primary amines was observed and, finally, a significant signal was indicated at 800 cm-1, which is associated with aliphatic hydrocarbons.
Effects of the botanical extracts of V. sphaerocephala on the growth of tomato plants
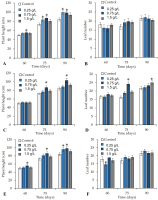
In the greenhouse experiment, the foliar application, direct application to the soil, and combined foliar + soil application of the V. sphaerocephala aqueous extract at the 0.25 g/L and 0.75 g/L concentrations significantly improved the growth parameters of tomato plants (p ≤ 0.05). Tomato plants treated with foliar sprays of V. sphaerocephala at the 0.75 g/L concentration showed a significant increase (p ≤ 0.05) in stem height on 60, 75, and 90 DAT (55.4, 86.9, and 98.4 cm, respectively) (Figure 3A). None of the concentrations of the V. sphaerocephala extracts resulted in significant differences in the number of leaves (Figure 3B).
Similarly, in tomato plants treated with the substrate applications of the V. sphaerocephala extract at 0.75 g/L, significant differences (p ≤ 0.05) were only observed in plant height at 75 and 90 d (86.7 cm and 103.2 cm), respectively, compared to the control (Figure 3C). Also, tomato plants that received the same V. sphaerocephala treatment at 0.75 exhibited significant differences (p ≤ 0.05) in the number of leaves (24) from the control (16 leaves). On the other hand, the application of the extract at the concentration of 1.5 g/L resulted in a significantly higher number of leaves than in the control but not until 90 DAT (24.9 and 21.7 leaves, respectively) (Figure 3D).
In addition, plants treated with both applications of V. sphaerocephala extract at the concentration of 0.75 g/L were higher than the control plants, i.e. their height was 55.6, 86.12, and 98.4 cm, respectively, on 60, 75, and 90 DAT (Figure 3E). The application of the V. sphaerocephala extract at the concentration of 0.25 g/L resulted in significantly higher plants (96.1 cm) than the control (84.3 cm) on 90 DAT (Figure 3E). No significant differences (p ≤ 0.05) were observed in the number of leaves between the treatments (Figure 3F).
Figure 3
Effect of the botanical extract of Verbesina sphaerocephala in (A, B) foliar application, (C, D) soil drench application, and (E, F) foliar + soil drench application on the growth tomato plant (height and number of leaves) on 60, 75, and 90 days after transplantation (DAT). Asterisk (*) indicates significant differences between the treatments and control.
Effects of the botanical extracts of V. sphaerocephala on the reproductive parameters of tomato plants
The foliar application of V. sphaerocephala significantly improved (p ≤ 0.05) various reproductive attributes of the treated plants on 75 and 90 DAT. The plants treated with V. sphaerocephala extracts at the 0.25% and 0.75% concentrations showed a significant increase in productivity in terms of the number of flowers (22.6 and 23.6 flowers, respectively, on 75 DAT) (Figure 4A). Beginning on 75 DAT, the number of fruits in plants treated with the V. sphaerocephala extract at the 1.5% concentration exhibited a significant (p ≤ 0.05) increase (average of 7.3 fruits per plant), compared to the control (average of 3.7 fruits per plant) (Figure 4B). On 90 DAT, we observed a significant increase (p ≤ 0.05) in the average number of flowers (31.1) in plants treated with the V. sphaerocephala extract at the 0.25% concentration (Figure 4A). In contrast, all plants that received the foliar application of V. sphaerocephala at the different concentrations (0.25, 0.75, and 1.5 g/L) exhibited significant increases (p ≤ 0.05) in the number of fruits (average values of 12.3, 14.2, and 13.1 fruits per plant, respectively) (Figure 4B).
The soil drench application of the V. sphaerocephala extract at 0.75% induced accelerated flower formation by 60 DAT. However, on 75 and 90 DAT, we observed a significant increase in the number of flowers in the plants treated with the V. sphaerocephala extract at 1.5 g/L (23 and 29.4 flowers, respectively), compared to the control (19.4 and 24.6 flowers) (Figure 4C). Regarding the number of tomato fruits, the applications of almost all the concentrations of V. sphaerocephala resulted in significant differences (p ≤ 0.05) with respect to the control on 75 and 90 DAT.
On 75 DAT, differences were only observed between plants treated with the V. sphaerocephala extracts at the concentrations of 0.25 g/L (5.8 fruits per plant) and 0.75 g/L (5.7 fruits per plant), compared to the control (3.7 fruits per plant) (Figure 4D). Similarly, on 90 DAT, all the concentrations of V. sphaerocephala extracts increased the number of tomato fruits (10.5, 11.0, 11.0, and 7.2).
The application of the V. sphaerocephala extract in combination (foliar + soil drench) at the concentrations of 0.25 and 1.5 g/L resulted in significant differences in the number of flowers. On 75 DAT, the application of V. sphaerocephala at the concentrations of 0.25 and 1.5 g/L resulted in a significantly greater number of flowers (22.8 and 22.9, respectively), compared to the control (19.4 flowers) (p ≤ 0.05). On 90 DAT, the same application of V. sphaerocephala (0.25 and 1.5 g/L) resulted in a significantly greater number of flowers (29.4 and 30.2 flowers, respectively), compared to the control (24.6 flowers) (p ≤ 0.05) (Figure 4E).
Finally, the foliar + soil drench application of the botanical extracts at all the concentrations significantly affected (p ≤ 0.05) the number of tomato fruits, compared to the control. After the application with 0.25, 0.75, and 1.5 g/L of V. sphaerocephala extracts, 5.8, 6.9, and 6.2 tomato fruits were recorded on 75 DAT. On 90 DAT, a two-fold increase in the number of tomato fruits was observed (12.6, 12.3, and 10.8 tomato fruits, respectively) following the application with 0.25, 0.75, and 1.5 g/L of V. sphaerocephala extracts (Figure 4F).
Regarding the yield variables, the 0.75 g/L V. sphaerocephala extract applied as foliar treatment resulted in the greatest number of fruits (14.5) (Table 4), as already mentioned in Figure 4B; however the weight per plant as the yield exhibited higher results in the V.s 1.5 g/L foliar treatment (82.6 g and 1.12 kg/plant, respectively). However, the V.s 0.75 g/L treatment applied as the foliar + soil drench variant is also a good option for future applications in tomato production.
Figure 4
Effect of botanical extracts from Verbesina sphaerocephala applied as (A, B) foliar application, (C, D) soil drench application, or (E, F) foliar + soil drench application on the reproductive parameters of tomato plants (flowers and fruit number) on 60, 75, and 90 days after transplantation (DAT). Asterisks (*) indicate significant differences between the treatments and control.
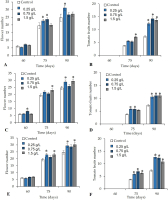
Table 4
Number of fruits per plant, fruit weight per plant and yield (kg/plant), under the influence of the different treatments of V. sphaerocephala (V.s).
Correlation between phytochemistry, application method, extract concentration, and growth parameters
A dendrogram classification based on the type of the botanical extract application (foliar, substrate, or foliar + substrate), the concentration (0.25, 0.75, and 1.5 g/L), and the presence of secondary metabolites resulted in the formation of four groups: (1) control plants without botanical extracts; (2) a group characterized by two subgroups (subgroup 1: plants treated with the botanical extract at 0.25 g/L; subgroup 2: plants-treated with the botanical extract via foliar or substrate application at 1.5 g/L); (3) a group of plants treated with the botanical extract at 0.75 g/L; (4) a group of plants treated with the foliar application at 1.5 g/L at a Euclidean distance of 3.2 (Figure 5A).
By relating the physicochemical variables via PCA (Figure 5B), we found that two factors explained 75.56% of the total variance. Factor 1 (PC1) explained 60.22% of the variance and was positively correlated with total phenols, proanthocyanidins, flavones and flavanols, flowers, and fruits. Factor 2 (PC2) explained 15.34% of the variance and was negatively correlated with phenolic acids and tannins and positively correlated with plant height, stem diameter, and leaf number (Figure 5B).
Figure 5
Hierarchical clustering and principal components analysis (PCA) used to elucidate treatment-variable relationships in the different botanical extracts from Verbesina sphaerocephala (V.s). The mean values of different parameters were clustered in four groups: (1) control; (2) plants treated with V. sphaerocephala at 0.25 g/L and V. sphaerocephala at 1.5 g/L; (3) plants treated with V. sphaerocephala at 0.75 g/L; (4) plants treated with foliar applications at 1.5 g/L. All data were analyzed via a principal component analysis (PCA). The lines originating from the central point of the biplots indicate positive or negative correlations of different variables; their closeness indicates the correlation strength with a particular treatment. The variables include the type of application and the growth and yield parameters.

. Discussion
The minerals detected in the aqueous extracts of V. sphaerocephala with nitrogen content, such as ammonia, are the preferred nutrients for plant growth. Bacteria found in the soil can convert ammonia into nitrite and nitrate, which are used by plants (González et al., 2009). Calcium is a non-mobile element within the plant and is added in the form of an aqueous extract to crops; this element is absorbed through root transpiration and thus from the substrate solution (Hancock et al., 2011). The extracts of V. sphaerocephala contain magnesium. The most important functions of magnesium in plant metabolism include photosynthesis and protein-starch synthesis, as well as other essential functions in nutrient absorption and carbohydrate translocation within the plant system (Ross, 2004). Phosphate, one of the elements found in the extracts of V. sphaerocephala, is a nucleic acid that activates the growth of meristematic tissues through the coenzymes NAD and NADP and plays an important role in oxidation-reduction reactions involving hydrogen transfer (Mixquititla-Casbis & Villegas-Torres, 2016). In previous studies of aqueous extracts of V. sphaerocephala applied to strawberries, nitrate and potassium were not detected (Velasco-Ramírez et al., 2022a). This could be due to the extracts being organic; plants can absorb these minerals through enzymatic activation utilizing various proteins contained in the extracts. However, the results may vary according to the phenological state of the leaves used to make the extracts and the analytical method of the study (Velasco-Ramírez et al., 2022b).
Effects of botanical extracts of V. sphaerocephala on the growth of tomato plants
From the phytochemical analysis of the extracts obtained from the leaves of V. sphaerocephala, it is clear that the secondary metabolites of the extracts acted as biostimulants. In fact, the secondary metabolites in the extracts tested in this study positively affected the physiology of tomato seedlings, similar to what was described by Velasco-Ramírez et al. (2021, 2022b), who applied V. sphaerocephala extract to cucumber seedlings. Although V. sphaerocephala extracts can improve root growth, they affect the germination time due to allelopathic responses (Velasco-Ramírez et al., 2022b).
Tomato production begins with germinating seeds in a protected environment with optimal greenhouse conditions. After germination, growth and development are sensitive. Therefore, seedlings should be transplanted to support plant productivity. Depending on the level of stress, plants can acclimatize to new environmental conditions to absorb water and nutrients efficiently (Vinković et al., 2022b2013). In the present study, we observed that the aqueous extract of V. sphaerocephala at the concentration of 0.75 g/L acted as a successful biostimulant, improving plant height and the number of leaves on 75 and 90 DAT, especially when applied to the substrate. However, group 3 of the PCA analysis (Figure 5) indicated that all three application methods (foliar, substrate, and foliar + substrate) significantly affected plant height. Likewise, the FT-IR spectrum (Figure 2) indicated the presence of amines in the V. sphaerocephala extract, which helped in the development of the plant and increased the yield thanks to the contribution of potassium and nitrogen.
The amount of reducing sugars in the V. sphaerocephala extracts facilitated the assimilation and transport of nutrient elements by the plant, likely by reducing osmotic pressure and thereby improving their entry into plant tissues. Thus, the sugars synthesized through biochemical reactions become part of the plant structure (e.g., cellulose) or reserve substances (e.g., starch) (Hernández-Bernal et al., 2022). Also, the flavonoid content in V. sphaerocephala extracts can promote the activation of nodulation genes, which favor nitrogen fixation and the attraction of pollinating insects, with all forms of application (Göttfert, 1993). Fernández et al. (2015) explained that a biostimulant applied to the leaves of crops is first adsorbed by the leaf surface, after which it penetrates the cuticle and is adsorbed and absorbed by the metabolically active cellular compartments of the leaf before being used. However, it is difficult to distinguish these processes from each other. Foliar application increases nutrient content in tissues, although the biological benefits of this form of application on the plant as a whole are often overlooked. Sujata et al. (2023) reported that agricultural productivity was negatively affected by drought stress and that biostimulants applied to the leaves of crops, such as Brassica juncea, increased the photosynthetic rate, stomatal conductance, and relative water content.
It is important to mention that V. sphaerocephala extracts have a high content of phenolic compounds, such as salicylic acid and caffeic acid (Jasso-de-Rodríguez et al., 2022) in addition to antioxidant activity, since they regulate homeostasis that leads to the ability to detect and control changes in the plant through the regulation of its internal environment to maintain adequate concentrations of ions, gases, and nutrients as well as the regulation of temperature, metabolism, and even its morphology (Peralta-Pérez & Volke-Sepúlveda, 2012). It has been shown that some phenolic compounds, such as salicylic acid, intervene in the production of proteins in plant cell tissues (Villanueva-Couoh et al., 2009), which is reflected in the strength and growth of the stem. On the other hand, V. sphaerocephala extracts also have high contents of flavonoids that encourage normal development by supporting the thylakoid membranes of chloroplasts, which are involved in the expression pathways of two multigenic enzymes, phenylalanine ammonium lyase and chalcone synthase, and constitute a group of important coloring substances in plants. However, the degree of transcription responds to the type and amount of biotic and abiotic stress (Faraa & Tahara, 1999).
Effects of botanical extracts of V. sphaerocephala on the reproductive parameters of tomato plants
The V. sphaerocephala extract with foliar application stimulated the greatest development of the number of flowers at the concentration of 0.25 g/L on 90 DAT; however, all three concentrations (0.25, 0.75, and 1.5 g/L) also significantly affected the number of fruits on 90 DAT. This agrees with Yari et al. (2022), who evaluated the application of a silicon-derived biostimulant on canola leaves and demonstrated that the number of pod grains, biomass, and grain yield increased significantly following the application. With the foliar + substrate application at the concentrations of 0.25 g/L and 1.5 g/L on 75 and 90 DAT, the increase in the number of flowers and the number of fruits was greater with the two concentrations. This result agrees with what was reported by Jasso-de-Rodríguez et al. (2020), who applied Rhus tumbari extract to tomato and reported increases in the number of flowers and fruits per plant.
The polyphenolic compounds of V. sphaerocephala promoted the highest yield, surpassing the control treatment. As mentioned previously, one of the compounds contained in the phenolic extract of V. sphaerocephala is salicylic acid (Jasso-de-Rodriguez et al., 2022). This compound participates in various physiological processes (e.g., flowering, root growth, and nutrient absorption at the cellular level), mitigates plant stress, and increases fruit yield and quality (Vázquez-Diaz et al., 2016). These results are interesting because the V. sphaerocephala extract contains flavonoids and phenolic acids (Velasco-Ramírez et al., 2021), as well as tannins, which interfere with the absorption of nutrients and anthocyanins, protect the plant against the effects of ultraviolet radiation and viral and microbial contamination, increase protection against biotic and abiotic stress, and give color to tomato fruits through anthocyanins located in discrete regions of the cellular vacuole called anthocyanoplasts (Pecket & Small, 1980) (Figure 5). This was also confirmed by the FT-IR spectrum (Figure 2) with the presence of alkenes, where ethylene, responsible for regulating different processes during fruit ripening, was mainly present.
The agronomic parameters of plant height, the number of flowers, and the number of fruits in this study responded positively to the application forms of the V. sphaerocephala extract. In particular, the foliar + substrate application appeared to further enhance the translocation of components from the aqueous extracts of V. sphaerocephala. The second application of the extract allowed the plants to tolerate stress in the form of temperature and agronomic management. Therefore, V. sphaerocephala leaves are potential candidates to produce botanical extracts and biostimulants to promote growth in tomato crops at low doses, especially when accompanied by low amounts. The application of these extracts provides a powerful ecological approach in crops of agricultural interest. The extracts acted as biostimulants, exerting an action similar to phytohormones in the growth and yield of tomato plants and inducing some changes in the physiological processes that improved plant growth and crop quantity and quality. This effect may be due to the individual action of a compound present in the extracts or to a synergism between polyphenolic compounds. We maintain that plant biostimulants derived from substances, such as alkaloids, terpenes, phenolic compounds, flavonoids, and isoflavonoids, positively affect plant growth and development when applied exogenously. As stated by du Jardin (2015), “biostimulants are defined by what they do, not by what they are.” More research is needed to determine what types of secondary metabolites (phenols and flavonoids) contained in V. sphaerocephala extracts improve plant development and to evaluate the effectiveness of the extracts on tomato yield and quality.
. Conclusions
The findings of this study demonstrate that botanical extracts from Verbesina sphaerocephala can effectively enhance the growth and yield of tomato plants in greenhouse conditions. The observed improvements in plant height, number of leaves, flowers, and fruits can be attributed to the bioactive polyphenolic compounds present in the extracts, which likely work synergistically to promote plant growth and stress resilience. These results highlight the potential of V. sphaerocephala extracts as sustainable biostimulants that can reduce dependence on chemical fertilizers and support sustainable agricultural practices. Future research should focus on elucidating the specific mechanisms of action of these bioactive compounds and evaluating the long-term effects of V. sphaerocephala extracts on various crops in different environmental conditions.