Introduction
Seed germination is the earliest and one of the most important processes in plant development. Its proper timing is crucial for future plant fitness and survival. Premature or delayed germination might lead to disturbances in development processes as well as to the exposure to stress conditions and decreased chances in intra- and interspecies competition for environmental resources. The germination process is divided into three phases of which the first phase is characterized by significant water uptake during the process of imbibition accompanied by early activation of metabolism and the second phase comprises mobilization of storage material, enhanced transcription, translation, and respiration, and increased growth of embryo cells. The final third phase includes further water uptake, seedlings growth, and final rupture of the seed coat and radical emergence (Wietbrecht et al.,2011). The course of the germination process is regulated by several internal and external factors. Internal regulation is mediated by plant hormones, namely abscisic acid, ethylene, auxins, gibberellins, cytokinins, and brassinosteroids and other signaling molecules including reactive oxygen species (ROS) and NO (Arc et al.,2013; Miransari & Smith,2014; Shu et al.,2016; Wojtyla et al.,2016). In the case of external factors, the main germination modulators include water status and light and temperature conditions (Donohue,2005; Finch-Savage & Leubner-Metzger,2006). However, the process might be similarly affected by environmental contaminants. Several studies have shown that exposure to metals such as Ni, Pb, Cd, and Cr decrease germination rate and/or hamper growth of young seedlings. The inhibitory effect is associated with disturbances in water uptake, oxidative stress, decreased level and activity of proteins, changes in membrane permeability, and DNA damage (Kranner & Colvillle,2011; Sethy & Ghosh,2013). In contrast, there are reports showing the stimulatory effect of metals at low concentrations on germination of several plant species (Ilić et al.,2015; Kavuličova et al.,2012; Lefévre et al.,2009). This phenomenon might be explained by hormesis – stimulation of growth by toxic ions at low concentration through, among other processes, perturbation in signaling network and activation of defense mechanisms (Poschenrieder et al.,2013). One of the important metal contaminants is Cd, which has been detected at elevated levels in soil samples from various regions of the world (Khan et al.,2011,2017; Wang et al.,2015). This highly mobile element is readily absorbed by plants leading to the development of severe toxicity symptoms including growth inhibition, disturbances in photosynthesis, and damage of proteins, membranes, and nucleic acids (Gallego et al.,2012; Khan et al.,2017; Tran & Popova,2013). Absorption of Cd by crops poses a threat to human health, as this metal is considered a class I carcinogen and causes alterations in structure and functioning of bones, kidneys, and lungs (Khan et al.,2017; Rizwan et al.,2017). The aim of the present study was to examine in detail the impact of Cd on the physiological state of soybean seeds during the earliest germination phase. The effect on seed cell viability, membrane integrity, intensity of oxidative stress, antioxidant properties, and germination rate are examined and discussed.
Material and Methods
Seeds Treatment and Growth Procedures
Soybean (Glycine max L. ‘Naviko’) seeds, kindly supplied by the Department of Genetics and Plant Breeding, University of Life Sciences in Poznań, Poland, were surface sterilized with 75% ethanol (5 min) and 1% sodium hypochlorite (10 min) and washed for 30 min under running water. Next, for every experimental treatment, 50 seeds were imbibed for 2 hr in 30 mL of distilled water (control) or CdCl2 (P.O.Ch.; Gliwice, Poland) with Cd at the concentration 5, 10, or 25 mg/L. After 2 hr, the seeds were washed thoroughly under running water and used for further analysis.
Measurement of Cd Uptake
Cd uptake by seeds was traced using atomic absorption spectrometry (AAS) (iCe 3000 series; Thermo Scientific, Waltham, Massachusetts, U.S.) with electrothermal atomization. AAS-Cd standard solution with a concentration of 1,000 mg/L was used for preparation of the standard (AAS standard solution; Merck, Kenilworth, New Jersey, U.S.). The quality control of AAS results was ensured using the standard reference materials 1570a (Trace Elements in Spinach Leaves) and 1575a (Pine Needles).
For analysis, approximately 0.2 g of dried sample was added to a Teflon vessel with 5 mL of concentrated HNO3 and 1 mL of H2O2. Subsequently, the samples were digested using a two-step temperature program. During the first step, the temperature was linearly increased to 160 °C for 15 min; the maximum power of the rotating magnetron was 400 W and pressure 2,000 kPa. During the second step, the temperature was maintained at 190 °C for 10 min. After digestion and cooling, digests were quantitatively transferred to 100-mL calibrated flasks and made up to the volume with double distilled water. The results are reported as the average of three repeated measurements, and all digestions were conducted in triplicate. All the reagents used for this study were of analytical grade.
For detection of Cd in the seeds, the seeds were cut in half, washed for 10 min in deionized water on a rocking platform and incubated for 30 min in 0.5% dithizone (Sigma-Aldrich; Saint-Louise, Missouri, U.S.) solution with 20 µL of ice acetic acid. In reaction to dithizone staining, tissues containing Cd acquire a pinkish-red color. Thereafter, the seeds were washed again with deionized water, dried with blotting paper, and photographed.
Evaluation of Cell Viability
Cell viability was evaluated based on two methods: Staining with Evans blue (EB; Sigma-Aldrich) and tetrazolium chloride (TTC) (Serva; Heidelberg, Germany). In the first method, based on the method described by Lehotai et al. (2011), with some modification, accumulation of EB indicates cell death. Seeds of similar fresh weight (200 mg) were incubated for 20 min in 0.25% EB solution, washed twice for 15 min in distilled water and homogenized using a mortar and pestle with destaining solution containing 50 mL of ethanol, 49 mL of distilled water, and 1 mL of 10% dodecyl sulfate (SDS; Serva). Samples were incubated in a heating block for 15 min at 50 °C and centrifuged for 15 min at 13,400 g. EB uptake was measured at λ = 600 nm using Biomate 3S spectrophotometer (Thermo Scientific). Destaining solution was used as the blank. Measurements were performed on samples from five independent experiments.
The second method of cell viability evaluation is based on the fact that in living cells dehydrogenase reduces TTC to insoluble red formazan (Towill & Mazur,1974). The seeds were weighed, transferred to Eppendorf tubes, and incubated with 1 mL of 0.4% TTC dissolved in phosphate buffet pH 7.5 for 24 hr at 20–22 °C in the dark. Thereafter, they were washed with distilled water, transferred to new Eppendorf tubes, supplemented with 0.5 mL of 95% ethanol and homogenized in Tissue Lyser II (Qiagen; Hilden, Germany). The samples were incubated for 1 hr at 55 °C, supplemented again with 0.5 mL of 95% ethanol, mixed on vortex, and centrifuged for 3 min at 10,000 g. The absorbance of the mixture containing 800 µL of supernatant and 1,400 µL of 95% ethanol was measured at λ = 485. The blank contained 95% methanol. Measurements were performed on samples from four independent experiments.
Examination of Membrane Integrity
Membrane integrity was estimated based on ion leakage. Fifty seeds were transferred to glass beaker for every experimental treatment and imbibed for 2 hr in distilled water or CdCl2 solutions on a shaker at 20–22 °C. Thereafter, the solutions were transferred to new beakers and their conductivity was measured using a conductometer (Tel-Eko; Wrocław, Poland). Measurements were performed on samples from four independent experiments.
Lipid Peroxidation
Oxidative stress intensity was estimated based on changes in the level of lipid peroxidation products, thiobarbituric acid reactive substances, according to Cuypers el al. (2011) with minor modifications. The seeds (200 mg) were homogenized using a mortar and pestle in 3 mL of 10% trichloroacetic acid (Sigma-Aldrich) buffer and centrifuged for 10 min at 12,000 g. Thereafter, 1 mL of supernatant was transferred to glass tubes, filled with 4 mL of 0.05% thiobarbituric acid (Sigma-Aldrich) dissolved in 10% trichloroacetic acid and incubated for 30 min in 95 °C. Afterwards, the samples were cooled, mixed by inversion, and centrifuged for 1 min at 5,000 g. The absorbance of the supernatant was measured at λ = 532 nm and corrected for unspecific absorbance at λ = 600 nm. Measurements were performed on samples from three independent experiments.
Measurements of Antioxidant Capacity
The antioxidant capacity was estimated based on the reduction rate of 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich), which changes color from dark violet to pinkish during the reduction process (Brand-Williams et al.,1995). The seeds (200 mg) were homogenized with 2 mL of 80% methanol, incubated for 2 hr at 37 °C on a shaker, and centrifuged for 10 min at 12,000 g. The absorbance of the solution containing 100 μL of sample, 250 μL of DPPH, and 2 mL of 80% methanol was measured spectrophotometrically at λ = 517 nm immediately after preparation and after 10 min of incubation in the dark. The blank contained 250 μL of methanol instead of DPPH. The antioxidant capacity is expressed as the difference between the absorbance before and after incubation in the dark. Measurements were performed on samples from four independent experiments.
Measurements of the Level of Nonenzymatic Antioxidants
The level of total soluble phenolics was measured using Folin–Ciocalteu (Merck) reagent according to Díaz et al. (2005). Seeds of similar weight (200–300 mg) were homogenized with 4 mL of 80% methanol, incubated for 15 min at 70 °C and after cooling, centrifuged at 2,500 g. The supernatant was diluted to 5 mL with 80% methanol. Mixtures containing 3,750 µL of distilled water, 250 µL of Folin–Ciocalteu reagent, and 250 µL of supernatant were prepared and supplemented after 3 min with 750 µL of 20% Na2CO3. Immediately afterwards, the absorbance of the prepared mixture was measured at λ = 760 nm.
The level of flavonoids was determined according to Jia et al. (1998). Seeds of similar weight (200–300 mg) were homogenized with 2 mL of 80% methanol, incubated for 2 hr at 37 °C, and centrifuged for 10 min at 13,400 g at 10–12 °C. Thereafter, mixtures containing 250 µL of supernatant, 1,250 µL of distilled water, and 75 µL of 5% NaNO3 (Merck) were prepared. After 5 min incubation at 20–22 °C, 150 µL of 10% AlCl3 (Merck) was added. Finally, after an additional 6 min of incubation at 20–22 °C, 500 µL of 1 M NaOH (Merck) was added and the absorbance of the mixture was measured at λ = 510 nm.
The level of anthocyanins was assessed using the method described by Sims and Gamon (2002) with some modifications. Seeds of similar weight (200–300 mg) were homogenized with a 1 mL mixture containing methanol/distilled water/HCl in the proportion 60:1:1. The samples were incubated for 24 hr at 4 °C. Thereafter, the samples were centrifuged at 13,400 g for 10 min and the absorbance of the supernatant was measured at two wavelengths: λ = 529 nm and λ = 650 nm. The level of anthocyanins was calculated according to the formula
Ascorbic acid level was measured based on the methods described by Mukherjee and Choudhuri (1985). Seeds of similar weight (200–300 mg) were homogenized with 2 mL of 5% trichloroacetic acid, transferred to glass tubes, and incubated for 15 min at 100 °C with 2 mL of 2% 2,4-dinitrophenylhydrazine (Sigma-Aldrich) dissolved in methanol and 40 µL of 10% thiourea (P.O.Ch) dissolved in 70% ethanol. The mixtures were cooled on ice and supplemented with 2.5 mL of 80% H2SO4 (P.O.Ch). Thereafter, the absorbance of the mixtures was measured at λ = 530 nm.
Measurements of Germination Rate and Growth Parameters
The seeds were transferred to glass Petri dishes, 30 cm in diameter, and covered with two layers of lignin and one layer of blotting paper. Then, they were watered with 30 mL of distilled water and kept in the cultivation room in the dark at 22 °C. The number of germinated seedlings was counted after 24, 48, and 72 hr. The roots of seedlings germinating for 72 hr were used for measurements of length and fresh and dry weight. Measurements were performed in five–six independent repetitions.
Statistical Analysis
Statistically significant differences between experimental treatments were calculated using the ANOVA test, at p = 0.05 using Free Statistics Calculators version 4.0 (https://www.danielsoper.com/statcalc/default.aspx). The statistically significant differences are denoted with an asterisk (*) on the graphs.
Results
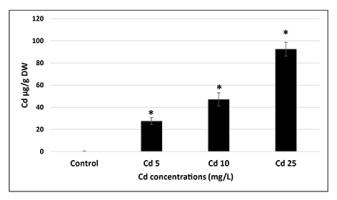
Even relatively short (2 hr) imbibition in Cd solutions resulted in significant (p = 0.05) uptake of this metal by the seeds. The level of Cd in the seeds was dependent on the applied metal concentration and reached nearly 100 µg/g dry weight in the case of the most severe treatment (25 mg/L) (Figure 1). Cd uptake by the seeds was also confirmed by histochemical staining with dithizone reagent (Figure S1). The images show that Cd is mainly accumulated in the cotyledons.
Figure 1
Cd content in the seeds imbedded for 2 hr in CdCl2 solutions with Cd at concentrations of 5 (Cd 5), 10 (Cd 10), and 25 (Cd 25) mg/L expressed in µg per g of dry weight (DW). The results are means of three independent repetitions ± SE. Significant differences (p = 0.05) in relation to the control are marked with an asterisk (*).
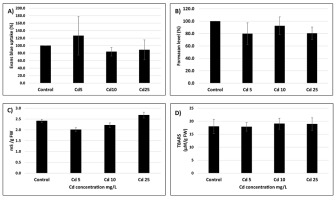
Despite significant uptake of Cd, the seeds showed no symptoms of metal toxicity. No differences were observed in the amount of dead cells assessed by two distinct methods – staining with EB and TTC assay (Figure 2A,B). The conductometric measurements of electrolyte leakage were similar in the control and Cd-treated seeds, implicating that the membrane integrity was intact (Figure 2C). Additionally, no symptoms of oxidative stress were observed as indicated by similar thiobarbituric acid reactive substances levels in all experimental treatments (Figure 2D).
Figure 2
Amount of dead cells expressed as % of the level of Evans blue (A) or tetrazolium chloride (B) changes in electrolyte leakage (C) and thiobarbituric acid reactive substances level (D) in seeds exposed to Cd at concentration 5 (Cd 5), 10 (Cd 10), and 25 (Cd 25) mg/L. The results are means of three–five independent repetitions ± SE. No significant differences were noted.
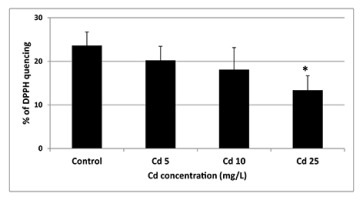
The only statistically significant difference (p = 0.05) between control and Cd-treated seeds was a decrease in the antioxidant activity noted in response to the highest applied Cd concentration (Figure 3).
Figure 3
Antioxidant activity of seeds exposed to Cd at concentration 5 (Cd 5), 10 (Cd 10), and 25 (Cd 25) mg/L expressed as % of DPPH quenching. The results are means of five independent repetitions ± SE. Statistically significant differences (p = 0.05) in relation to the control are marked with an asterisk (*).
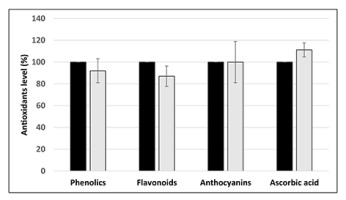
However, the level of several nonenzymatic antioxidants, namely total soluble phenolics, flavonoids, anthocyanins, and ascorbic acid, did not differ between the control and the seeds treated with Cd at the concentration 25 mg/L (Figure 4).
Figure 4
Level of nonenzymatic antioxidants in control seeds (black bars) and seeds treated with Cd at the concentration 25 mg/L (light greybars) expressed as % in relation to the control. The results are means of three–four independent repetitions ± SE. No significant differences were noted.
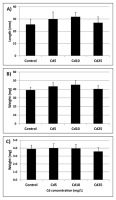
Seeds from all experimental treatments showed a similar germination rate measured at three time points – after 24, 48, and 72 hr (Figure 5). The germinated seedlings showed no differences in the terms of root length and fresh and dry weight (Figure 6A–C).
Figure 5
Germination rate of control seeds (black bars) and seeds preincubated in Cd solutions with Cd at concentration 5 mg/L (white bars), 10 mg/L (grey bars), and 25 mg/L (creamy bars) measured after 24, 48, and 72 hr. The results are means of six independent repetitions ± SE. No significant differences were noted.
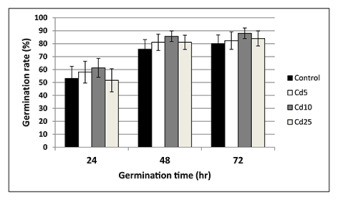
Figure 6
Root length (A), fresh weight (B), and dry weight (C) of seedlings grown from seeds preincubated in Cd solution with Cd at concentrations of 5 (Cd 5), 10 (Cd 10), and 25 (Cd 25) mg/L measured after 72 hr of germination. The results are means of five independent repetitions ± SE. No significant differences were noted.
Discussion
High Cd levels have been detected in various regions of the world (Khan et al.,2011,2017; Wang et al.,2015). This metal is readily taken up by plants thereby altering their functioning and limiting growth. Importantly, Cd absorbed by edible plants can enter the human food chain (Rizwan et al.,2017). According to the Agency for Toxic Substances and Disease Registry (2020), Cd is among the top 10 substances which pose the most significant threat to human health.
The present study assessed the impact of Cd on the germination process in an important crop plant species – soybean. The results show that even short-term imbibition in Cd solution results in significant uptake of this element by soybean seeds (Figure 1). The amount of Cd reached nearly 100 µg/g of dry weight in the case of the treatment with the highest applied metal concentration (25 mg/L). However, metal treatment did not affect cell viability assessed by two distinct methods – staining with EB and TTC assay (Figure 2A,B). Additionally, seeds imbibed in metal solutions showed no differences in the level of electrolytes leakage or lipid peroxidation products (thiobarbituric acid reactive substances) (Figure 2C,D). These results indicate that the cellular membranes were not damaged under Cd treatment.
In contrast, imbibition in the highest Cd concentration (25 mg/L) led to significant decrease in seeds antioxidant activity (Figure 3). The fact that Cd affects antioxidant machinery is well documented. However, so far, its impact on the antioxidant system has been examined mainly in seedlings or fully developed plants (reviewed by Chmielowska-Bąk et al.,2014). In the present study, we show that Cd already decreases antioxidant capacity in seeds during the earliest germination phase. Further investigation showed that the observed attenuation of antioxidant activity is not associated with the decrease in the level of major nonenzymatic antioxidants including total soluble phenolics, flavonoids, anthocyanins, and ascorbic acid (Figure 4). Therefore, it can be suspected that the main reason for the observed depletion in the antioxidant defense are changes in the activity of antioxidant enzymes. This assumption would need further evaluation.
According to the “oxidative window for germination” hypothesis, precise regulation of ROS levels, among others by the antioxidant system, is indispensable for the proper progression of the germination process (Bailly et al.,2008). The beneficial roles played by ROS during germination include involvement in signaling network, selective oxidation of transcripts and proteins associated with mobilization of storage material, endosperm weakening, and enhancing protection against pathogens. In turn, ROS overproduction is associated with loss of membrane integrity, DNA damage, aging, and decreased seeds vigor (Bailly et al.,2008; Waterworth et al.,2015; Wojtyla et al.,2016). Therefore, it could be expected that the observed decrease in antioxidant activity in Cd-treated soybean seeds will affect their future germination. However, no perturbations in the germination process were observed. Preincubation in Cd did not affect germination rate at any of the measured timepoints – after 24, 48, or 72 hr (Figure 5). Additionally, seedlings grown from Cd pretreated seeds showed no differences in any of the measured growth parameters (Figure 6A–C). The effect of Cd treatment on germination rate and growth of young seedlings varies depending on plant species, applied concentration, duration of treatment, and method of metal application. For instance, a study on six species of pulses showed that in general, metal treatment inhibited germination rate and led to decrease in root and shoot length (Tao et al.,2015). Similarly, treatment of pigeon pea, fenugreek, and maize with Cd led to reduced germination rate and/or seedlings growth (Sneideris et al.,2015; Wahis et al.,2015; Zayneb et al.,2015). However, in the case of flax and China aster the inhibitory effect was observed only at the highest metal concentrations (Kavuličova et al.,2012). Moreover, in Dorycnium pentaphyllum application of Cd at low concentration resulted in slightly higher final germination percentage, although severe Cd stress led to drastic disturbances in germination (Lefévre et al.,2009).
In summary, it can be concluded that although cadmium is considered a highly toxic compound, soybean seeds are tolerant to its action, at least during the first phase of germination. The only observed difference between control seeds and seed exposed to Cd was a decrease in the total antioxidant activity observed in response to the highest metal concentration. It would be interesting to assess if the early exposure to the metal affects future development or stress response of soybean plants.
Authors’ Contributions
JCB and JD participated in the design of the research; MF and IZ carried out the measurement of Cd content; RH and JCB carried out the experiments concerning seeds physiological state; JCB and SI conducted measurements of germination rate and seedlings growth; JCB, MF, IZ, and JD analyzed the data; JCB wrote the manuscript


