Abbreviations
GLA: glutaraldehyde
MS: Murashige and Skoog medium
NOCIM: non-organogenic callus induction medium
OCIM: organogenic callus induction medium
PAS: periodic acid–Schiff
PBS: phosphate-buffered saline
PGR: plant growth regulator
TBO: toluidine blue O
TEM: transmission electron microscopy
TDZ: thidiazuron
2,4-D: 2,4-dichlorophenoxy-acetic acid
. Introduction
Endosperm is a specific storage tissue in angiosperms that supports the development of the embryo. In many species, the endosperm has a higher ploidy level than the maternal tissue and the embryo (Baroux et al., 2002). The plant tissues show high potency to modify their metabolism and development and consequently - their fate (Morinaka et al., 2023). Tissue culture conditions allow the enhancement of this ability, which can lead to dedifferentiation and plant regeneration. Plants regenerated from endosperm can show a higher ploidy level, the same as endosperm. However, endosperm is known to be recalcitrant to dedifferentiation, even when cultured in vitro. In addition, there is no evidence that the fate of endosperm changes under natural conditions. Regardless, we have established a protocol for callus proliferation and shoot induction in the isolated endosperm of the kiwiberry, Actinidia arguta (Abdullah et al., 2021; Popielarska-Konieczna et al., 2020), which is a breakthrough result in terms of dealing with recalcitrant (to dedifferentiation) tissues.
Obviously, the dedifferentiation process into the callus stage, called proliferating cell mass (Fehér, 2019), depends on the kind of tissue and laboratory conditions (Efferth, 2019). Previous reports, including histological analyses of the dedifferentiation process under tissue culture conditions, have concerned mainly mesophyll and epidermal cells of leaf, hypocotyl, or cotyledon as the initial explants and have described the vacuolization, nucleus position, and intercellular spaces in particular (Agrawal et al., 1989; Anzidei et al., 2000; Corredoira et al., 2006; Kim et al., 2021; Morinaka et al., 2023 and citations therein; Suzuki et al., 2002). The pattern of starch accumulation within callus tissue has been reported for the induction of meristematic and somatic embryogenesis (Anzidei et al., 2000; Corredoira et al., 2006; Fernando et al., 2003). Some histological studies did not examine the presence of starch granules under callus formation and morphogenesis (Agrawal et al., 1989; Kim et al., 2021; Okello et al., 2021; Suzuki et al., 2002).
Our previous observations (Abdullah et al., 2021) showed that isolated endosperm in kiwiberry cv. Bingo cultured on NOCIM and supplemented with 2,4-dichlorophenoxy-acetic acid (2,4-D) and kinetin resulted in callus induction with cell proliferation only, with no organogenic events like adventitious shoot or root formation, even after six months of culturing. The efficiency for callus induction on NOCIM was 0.97. On the OCIM supplemented with 0.5, 0.25, and 1.0 mg/l of thidiazuron (TDZ), the efficiency of callus induction was 0.85, 0.95, and 0.99, respectively. In contrast to the NOCIM, on OCIM the growth of induced callus gave rise to adventitious shoots after six weeks of culture with a probability of p = 0.22 for 0.5 mg/l of TDZ. A previous histological study performed on endosperm-derived calli tissue in kiwiberry was conducted after six weeks of the culture when the features distinguishing explants on OCIM and NOCIM with different competency to adventitious shoot formation were clearly visible (Popielarska-Konieczna et al., 2020).
In this study, we analyzed the ultrastructural differences of cells undergoing two pathways – organogenic and non-organogenic – which can be detected from culture with conditions for organogenic or non-organogenic callus induction and proliferation. The observations were taken after one, two, three, and four weeks of the culture on OCIM and on NOCIM. One visible feature was the appearance of amyloplasts during cell dedifferentiation and proliferation, and this depended on the time period and the developmental pathway. This is the first report concerning the initial stages of the development of isolated endosperm entering an organogenic or non-organogenic pathway. The presented data complete knowledge about plant cell organization during dedifferentiation under different treatments.
. Material and methods
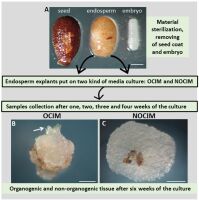
The study was based on the plant material and experimental system (Figure 1) described previously (Abdullah et al., 2021; Popielarska-Konieczna et al., 2020). The mature fruits (soft, ready-to-eat) of kiwiberry cv. Bingo were harvested from the plant germplasm collection at the Warsaw University of Life Sciences (SGGW) in Warsaw, Poland. The fruits were sterilized and then the seeds were taken out. Following the published protocol, endosperm explants were obtained after the seed coat and embryo were dissected (Abdullah et al., 2021; Popielarska-Konieczna et al., 2020). The explants were put on 60 mm Petri dishes with a culture medium based on Murashige and Skoog (1962) macro- and microelements, vitamins (Duchefa), and the addition of 3% sucrose, 0.6% agar (Duchefa), and PGRs. Two kinds of media were used: the first was supplemented with 0.5 mg/l of thidiazuron (TDZ), defined as the organogenic callus induction medium (OCIM), and the second, supplemented with 2.0 mg/l of 2,4-D and 5.0 mg/l of kinetin, was defined as the non-organogenic callus induction medium (NOCIM). Observations were performed and images were recorded using a dissecting binocular microscope (Zeiss Stemi SV 11, Germany) equipped with a digital camera (Canon EOS 760D).
Figure 1
Experimental model of the culture of isolated endosperm in Actinidia arguta cv. Bingo. (A) Seed coat and embryo were removed from the seed and the endosperm explants were placed on OCIM or NOCIM media. Samples were collected after one, two, three and four weeks of culturing. (B) and (C) Explants after six weeks of the culture show visible differences in morphology, such as the induction of shoot buds (arrow) on the OCIM. Scale bars: 200 µm (A); 500 µm (B).
For the histological and ultrastructural analyses, the freshly isolated endosperm and samples were collected after one, two, three, and four weeks of the culture on OCIM and NOCIM. For the histological analysis, the samples were fixed in 5% (w/v) glutaraldehyde (GLA) in 0.1 M PBS (pH 7.2), embedded in synthetic resin Technovit 7100, and then cut and stained with 0.01% (w/v) toluidine blue (TBO) or the periodic acid–Schiff (PAS) reaction. Sections were observed with bright-field illumination using a microscope (Nikon Eclipse E400). For the transmission electron microscopy (TEM) analysis, the samples were prefixed in 2.5% (w/v) GLA and 2.5% (w/v) in 0.1 M cacodylate buffer (pH 7.0). The samples were post-fixed in buffered 1% (w/v) osmium tetroxide (OsO4), then were treated with 1% (w/v) uranyl acetate, dehydrated in a graded acetone series, and embedded in Spurr’s resin. The sections were stained with uranyl acetate and lead citrate and were examined with a Philips CM 100 electron microscope.
. Results
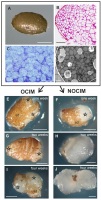
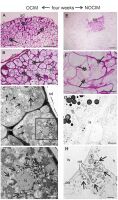
The figures show representative images selected from an average of between ten to twenty sections per OCIM and NOCIM conditions and each time period of the culture. The freshly isolated endosperm was still covered with the inner part of the seed coat, visible as a light brown layer on the explant (Figure 2A). Histological and ultrastructural analysis of freshly isolated endosperm revealed storage tissue composed of tightly arranged cells (Figure 2B–C) with abundant protein and lipid bodies (Figure 2D). These remnants of seed coat were visible during the growing of the explant, even after four weeks of the culture on OCIM and on NOCIM (Figure 2E–J). After one week of the culture, the explants on both media showed a similar appearance (Figure 2E–F), with the swollen regions of cells that had responded to the culture conditions. After two weeks, the explants on OCIM had formed smaller and more compact tissue (Figure 2G) than tissue cultured on NOCIM (Figure 2H). The explants developing on OCIM and NOCIM could easily be distinguished from each other after four weeks of the culture. We observed a compact callus with bulged domains on the OCIM (Figure 2I), in contrast to the more expansive and loosely arranged callus on the NOCIM (Figure 2J).
Figure 2
Fresh isolated endosperm (A–D). General view (A), histological section stained with PAS (B) and TBO (C) and ultrastructural (D) image. Note the cells filled with storage substances, lipid (lb) and protein (pb) bodies, and the lack of amyloplasts and starch granules. Explants after one (E, F), two (G, H), and four (I, J) weeks on OCIM and NOCIM. There are visible remnants of the inner part of seed coat (sd) and swollen responsive parts of explants (asterisks). Scale bars: 2 µm (D); 50 µm (C); 100 µm (B); 500 µm (A, E–J).
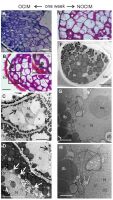
After one week of the culture on OCIM, dedifferentiated cells were detected, especially near the surface of the explants (Figure 3A–B). The sections stained through the PAS reaction showed a limited number of starch granules (Figure 3B1). This observation was confirmed by ultrastructural analysis, where amyloplasts with starch granules were indicated (Figure 3C–D). The number of protein and lipid bodies decreased significantly and other organelles were visible, such as nuclei or vacuoles (Figure 3C–D). After one week of the culture on the NOCIM, the growing part of the explant possessed modified cells of endosperm, regressing storage substances (washed-out pink after the PAS reaction), and no visible starch granules (Figure 3E). The electronograms revealed the presence of lipid and protein bodies (Figure 3F–G) and organelles that were not visible in the freshly isolated endosperm, such as nuclei, mitochondria, and plastids (Figure 3G–H). The plastids did not contain starch granules (Figure 3G–H).
Figure 3
Analysis of endosperm explants of Actinidia arguta cv. Bingo on OCIM (A–D) and NOCIM (E–H) after one week of the culture. Histological sections stained with TBO (A) and PAS (B, E) and ultrastructural images (C, D, F–H); (B1) magnified part of (B) (frame); (A–D) the dedifferentiated cells (dashed line) and starch granules dyed in purple (double arrows) in the OCIM explants. Amyloplasts with starch granules (arrows); lobed nucleus (N) with nucleolus (nu); lipid bodies (lb); cell wall (cw), and vacuoles (v); (E) cell walls dyed in purple (white asterisks) and faded color of storage substances (dotted lines); (G, H) in the cytoplasm visible mitochondria (mt) and the plastids (pl) with inner membranes; the inner seed coat (sd). Scale bars: 1 µm (H); 2 µm (G); 5 µm (C, D, F); 25 µm (B1); 50 µm (A, B, E).
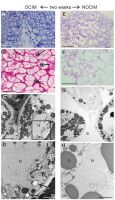
After two weeks of the culture, the histological and ultrastructural analyses revealed distinct differences in the responsive parts of the explants on OCIM and NOCIM (Figure 4A–H). The compact arrangement of cells and the presence of starch granules in the tissue on OCIM (Figure 4A–B) contrasted with the loosely attached cells cultivated on NOCIM (Figure 4E–F). Additionally, the explants on NOCIM showed no starch granules after the PAS reaction (Figure 4F). The electronograms confirmed the presence of amyloplasts under the OCIM condition (Figure 4C–D) and an absence of starch granules in the plastids of the tissue cultured on NOCIM (Figure 4G–H). The lack of amyloplasts and starch granules was also observed for the NOCIM-treated culture after three weeks (Figure S1).
Figure 4
Analysis of endosperm explants of Actinidia arguta cv. Bingo on OCIM (A–D) and NOCIM (E–H) after two weeks of the culture. Histological sections stained with TBO (A, E) and PAS (B, F) and ultrastructural images (C, D, G, H); (D) magnified part of (C) (frame). Note the starch granules (double arrows), amyloplasts with starch granules (arrows), lobed nucleus (N), lipid bodies (lb), and cell wall (cw); (G) large intercellular spaces (is); (H) plastids (pl) with thylacoids and mitochondria (mt). Scale bars: 1 µm (D); 2 µm (C, H); 5 µm (G); 50 µm (A, B); 100 µm (E, F).
After four weeks of the culture, the explants showed a continuation of the different tissue organization, which was detected at earlier stages of the cultivation on OCIM and NOCIM (Figure 5A–H). The callus cells on OCIM were in close contact, and abundant occurrence of amyloplasts in cells was observed (Figure 5A–D). In contrast, a limited number of starch granules for the scarce parts of the callus were observed for NOCIM (Figure 5E–H). The development of intercellular spaces was also noticed (Figure 5G).
Figure 5
Analysis of endosperm explants of Actinidia arguta cv. Bingo on OCIM (A–D) and NOCIM (E–H) after four weeks of the culture. Histological sections stained with PAS (A, B, E, F) and ultrastructural images (C, D, G, H); (D) magnified part of (C) (frame). Note the starch granules (double arrows), amyloplasts with starch granules (arrows), nucleus (N) with nucleolus, lipid bodies (lb), mitochondria (mt), remnants of isolated endosperm (en) and inner seed coat (sd), cell wall (cw), cells with phenolic deposits (ph), and intercellular spaces (is); (C) thin, newly arranged cell walls (full arrows) are visible. Scale bars: 2 µm (D, G, H); 5 µm (C); 50 µm (B, F); 100 µm (A, E).
. Discussion
A compact versus loose arrangement of cells is one of the most characteristic features of morphogenic competency of callus (Morinaka et al., 2023 and citations therein). In this study, differences in the arrangement of endosperm-derived cells were observed after two weeks of the culture. Moreover, the amyloplasts and starch granules were observed in explants after one week of the culture on OCIM, whereas in the callus cultivated on NOCIM, it was not until the fourth week of the culture that amyloplasts were detected. We suggest that the occurrence of amyloplasts, their number, and the time when they appear may be markers of cells following the organogenic pathway.
The preparation of cells for organogenic events can be manifested by the accumulation of storage substances, which can be easily converted into energy or be involved in metabolic pathways. This has been suggested in reports concerning callus proliferation and shoot induction (Carciofi et al., 2012; Czernicka et al., 2021; Lee & Huang, 2019; Santos et al., 2018). The strong correlation between starch accumulation and organ development was prominent in many different systems (Fortes & Pais, 2000; Karim et al., 2006; Thorpe & Murashige, 1968), indicating a gradual gathering before any observable organ formation and further utilization of the storage material in the organogenic process. Histological analysis of isolated endosperm was conducted only for the organogenic pathway for Passiflora cincinatta (Silva et al., 2020). Although there was a lack of information about the type of storage organelles, biochemical analysis revealed lower total lipid and soluble protein content in endosperm explants from the first day of the culture. However, the content of carbohydrates was higher. In this study, it was noted that the number of lipid and protein bodies decreased subsequently during the culture. Additionally, organ-forming cultures showed higher amounts of metabolites such as starch, but also exhibited higher enzyme activities than non-organ-forming cultures (Naidu & Kishor, 1995). In our recent research, we reported that the isolated endosperm in cv. Geneva showed a significantly lower efficiency in inducing adventitious shoots in comparison with cv. Bingo (Abdullah et al., 2021), even when cultivated on OCIM medium. Ultrastructural analysis of endosperm explants of cv. Geneva revealed a later appearance of starch granules in comparison with the endosperm-derived callus of cv. Bingo (data unpublished). These data suggest that the reorganization of isolated endosperm cells is manifested by the utilization of storage substances such as lipid or protein bodies, as well as the appearance of amyloplasts during dedifferentiation and the initial stages of proliferation of endosperm-derived cells. These changes are related to the type of developmental pathway: organogenic or non-organogenic.
. Conclusions
The dedifferentiation processes for OCIM and NOCIM were similar at the morphological level for the first week of the culture. A limited number of amyloplasts appeared in the cells after one week on OCIM, and in subsequent weeks an abundance of amyloplasts with starch granules were detected for this treatment. Limited starch granules were observed in the cells on NOCIM condition after four weeks of the culture. The amount and especially the timing of appearance of amyloplasts are distinct features between endosperm undergoing the two treatments of cultivation, OCIM and NOCIM, which lead to the formation of callus with organogenic or non-organogenic competency.
. Supplementary material
The following supplementary material is available for this article:
Figure S1. Analysis of endosperm explants of Actinidia arguta cv. Bingo on NOCIM (A–D) after three weeks of the culture. Histological sections stained with PAS (A–C) and ultrastructural image (D). Note the remnants of isolated endosperm (en), cell wall (cw), intercellular space (is), cells with phenolic deposits (ph), and lipid bodies (lb). Scale bars: 5 µm (D); 50 µm (C); 100 µm (A, B).


