. Introduction
Significance and Major Advantages of Plant In Vitro Cultures
Plants are the exclusive source of flavors, fragrances, dyes, pigments, pesticides, food additives, and life-saving drugs for the majority of the global population. More than 80% of the approximately 30,000 known natural products are of plant origin. The use of plant cells for the production of natural or recombinant compounds of commercial interest has gained attention over the past few decades. Secondary metabolites (also called specialized metabolites) play a major role in the adaptation of plants to their environment and are an important source of pharmaceuticals (Alamgir, 2017; Hussain et al., 2012).
Most pharmaceutically important natural products are isolated from wild or cultivated plants, because of their highly complex structures that make their chemical synthesis not economically feasible (Oksman-Caldentey & Inzé, 2004). However, such methods consume more plant resources than nature could actually regenerate. Additionally, obtaining sufficient active substances from plants is challenging because their content is generally low, plant growth is often slow and requires special cultivation conditions, and some species is restricted to certain climatic zones.
Plant in vitro cultures, since their first use (Haberlandt, 1943), have opened new opportunities for their use and have become a tool in both basic and applied research. They have enabled mass clonal propagation of the most valuable or endangered species independent of their natural life-cycle under strictly controlled conditions. They have also been used as tools for introducing genetic modifications. Moreover, they have increased the acquisition of biologically active compounds (Altpeter et al., 2016; Debnath et al., 2006; Karuppusamy, 2009; Thorpe, 2007).
Biotechnological production of active compounds in plant cell and organ cultures is an attractive alternative; however, the commercial success is limited till date because of insufficient understanding of their biosynthesis mechanism. Advances in plant genomics and metabolite profiling offer possibilities for studying the complexity of the biochemical capacity of plants. Genomics tools can be used to enhance the production of known target metabolites or synthesize novel compounds using combinatorial biochemistry in cultivated plant cells (Oksman-Caldentey & Inzé, 2004).
Despite some limitations, research in plant tissue and organ culture technology has produced many bioactive metabolites (alkaloids, terpenoids, steroids, saponins, phenolics, flavonoids, and amino acids) for new therapeutics under defined conditions (Alamgir, 2017; Hussain et al., 2012).
Since the 1960s, intensive research has introduced in vitro tissue culture-derived secondary metabolites, including scopolamine, rosmarinic acid (RA), shikonin, and berberine. Paclitaxel is a flagship example of using in vitro cultures to extract valuable therapeutic substances. Obtaining paclitaxel from naturally growing yew trees is economically and ecologically disadvantageous due to the long life-cycle of Taxus spp. Additionally, the chemical synthesis of paclitaxel is difficult and results in significant yield losses at almost every stage. Biotechnological studies reported biosynthesis of both paclitaxel and its precursor baccatin III from Taxus brevifolia suspension cultures. The in vitro synthesis of bioactive compounds or their precursors not only controls microbiological purity, but also allows the production of the given compound independently of its natural sources as shown in the example above. Furthermore, biosynthesis of secondary metabolites is carried out in a predictable, reproducible, and tightly controlled manner. The application of elicitors, appropriate culture medium, genetic modifications, and selection of suitable culture methods, including cell immobilization or large-scale farming in bioreactors, improves the efficiency of biosynthesis (Michalak, 2021).
Unfortunately, the number of secondary metabolites commercially extracted from tissue and cell cultures for pharmaceutical purposes remains low primarily due to the high demands of the industry, both in terms of the efficiency of the processes, as well as the purity and quality of the ingredients and products. However, this does not mean that there is little interest in plant in vitro culture. Innovations and developments in other industries, particularly cosmetics, increase the demand for plant origin components. Increasing consumer awareness, concern for sustainability and the environment, and the desire to use effective and safe products, often with ingredients of exotic origins (including endemic plants), will expand the use of in vitro plant cultures (Fonseca-Santos et al., 2015).
This review presents the achievements of in vitro plant culture studies for the production of bioactive compounds in scientific centers in Poland with a pharmaceutical profile. The main research findings are summarized in Table 1.
Table 1
Summary of secondary metabolites obtained from various in vitro-cultured plant tissues.
| Family/Species | Type of in vitro culture | Secondary metabolite | Research center* | References |
|---|---|---|---|---|
| Apiaceae | ||||
| Ammi majus | Callus | Umbelliferone, bergapten, xanthotoxin, isopimpinellin, imperatorin | 3 | Ekiert, 1993, 2004; Ekiert & Gomółka, 2000a |
| Ammi majus | Callus | Umbelliferone | 4 | Królicka et al., 2001 |
| Ammi majus | Cell suspension | Umbelliferone | 4 | Królicka et al., 2001 |
| Ammi majus | Hairy roots | Umbelliferone | 4 | Królicka et al., 2001 |
| Ammi majus | Callus | Scopoletin, dehydrogeijerin | 4 | Staniszewska et al., 2003 |
| Eryngium planum | Micropropagated plants (shoots and roots), shoot culture, callus, cell suspension | Rosmarinic acid, chlorogenic acid, caffeic acid | 2 | Kikowska et al., 2012, 2015; Thiem et al., 2013 |
| Eryngium planum | Micropropagated plants (shoots and roots), root culture, callus, cell suspension | Triterpenoid saponins: 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl-21-O-acetyl,22-O-angeloyl-R1-barrigenol [ES1], 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl-22-O-angeloyl-R1-barrigenol [ES2], 3-O-β-d-glucopyranosyl-(1→2)-β-d-glucuronopyranosyl-22-O-angeloyl-A1-barrigenol | 2 | Kikowska et al., 2019; Kowalczyk et al., 2014 |
| Pastinaca sativa | Callus | Umbelliferone, bergapten, xanthotoxin, isopimpinellin | 3 | Ekiert, 2004; Ekiert & Gomółka, 2000b |
| Asteraceae | ||||
| Cichorium intybus | Hairy roots | 8-Deoxylactucin, lactucopicrin and crepidiaside B, sonchuside A, and zaluzanin-like guaianolide – ixerisoside D, (7S,8R)-3′-demethyl-dehydrodiconiferyl alcohol-3′-O-β-glucopyranoside, 3,5-DCQA and 8-deoxylactucin glucoside, hydroxycinnamates | 7 | Malarz et al., 2002, 2007; Malarz, Stojakowska, & Kisiel, 2013; Malarz, Stojakowska, Szneler, & Kisiel, 2013; |
| Lactuca aculeata | Callus | Mono- and dicaffeoylqiunic acids, 1,5-di-O-caffeoylquinic acid, 1,5-DCQA, (+)-5-methoxybalanophonin, syringaresinol, (+)-buddlenol A, (+)-balanophonin-9-O-β-glucopyranoside, (−)-dihydrodehydrodiconiferyl alcohol-4-O-β-glucopyranoside, (−)-dihydrodehydrodiconiferyl alcohol-9-O-β-glucopyranoside | 7 | Stojakowska & Malarz, 2017 |
| Lactuca virosa | Hairy roots | 8-Deoxylactucin, jacquinelin, crepidiaside B and picriside A, macrocliniside A, ixerin F and scorzoside, lactuside A, lactucopicrin, 5-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid | 7 | Kisiel et al., 1995; Stojakowska et al., 2012 |
| Lactuca virosa | Callus and cell suspensions | Neolignans, caffeic acid derivatives | 7 | Stojakowska & Kisiel, 2000; Stojakowska et al., 2012 |
| Boraginaceae | ||||
| Cynoglossum columnae | Anatomical roots | Rinderol, cynoglosol | 1 | Jeziorek et al., 2019 |
| Rindera graeca | Hairy roots | Rinderol | 1 | Graikou et al., 2021 |
| Byblidaceae | ||||
| Byblis liniflora | Plantlets | Acteoside, isoacteoside, desrhamnosylacteoside, desrhamnosylisoacteoside | 2 | Schlauer et al., 1994 |
| Droseraceae | ||||
| Dionaea muscipula | Plantlets | Plumbagin, 8,8′-biplumbagin, 3-chloroplumbagin, maritinone, ellagic acid, 3-O-methylellagic acid, 3,3′-di-O-methylellagic acid, 3,3′-di-O-methylellagic acid 4-O-glucoside, 3,3′-di-O-methylellagic acid 4,4′-di-O-glucoside, kaempferol 3-O-glucoside and galactoside, quercetin 3-O-glucoside and galactoside, 2″-O-galloyl esters of quercetin 3-O-glucoside and galactoside; 1-O-galloylglucose. Plumbaside A | 2 | Kukułczanka & Budzianowski, 2002; Pakulski & Budzianowski, 1996a, 1996b |
| Dionaea muscipula | Adventitious shoots | Plumbagin | 4 | Krolicka et al., 2008 |
| Dionaea muscipula | Transformed teratomas | Plumbagin | 4 | Makowski et al., 2021 |
| Droseracapensis var. alba | Transformed teratomas | Ramentaceone | 4 | Krolicka et al., 2010 |
| Drosera capensis | Adventitious shoots | Ramentaceone | 4 | Krolicka et al., 2008 |
| Drosera gigantea | Leaf rosettes | Plumbaside A, plumbagin, droserone (3,5-dihydroxy-2-methyl-1,4-naphthoquinone), hydroxydroserone (3,5,8-trihydroxy-2-methyl-1,4-naphthoquinone), droserone 5-O-glucoside, hydroxydroserone 5-O-glucoside | Budzianowski, 2000 | |
| Drosera intermedia | Leaf rosettes | Myricetin 3-O-rhamnoside, quercetin 3-O-rhamnoside, quercetin 3-O-galactoside, quercetin 3-O-glucoside, kaempferol 3-O-galactoside, kaempferol 3-O-glucoside, plumbaside A (2-methyl-1,4,5-trihydroxynaphthalene 4-O-β-glucopyranoside), rossoliside, plumbagin, 7-methyljuglone | 2 | Budzianowski, 1996; Budzianowski et al., 1993 |
| Drosera rotundifolia | Leaf rosettes | Rossoliside | 2 | Budzianowski, 1996 |
| Drosera spatulata | Leaf rosettes | Myricetin 3-O-rhamnoside, quercetin 3-O-rhamnoside, quercetin 3-O-galactoside, quercetin 3-O-glucoside, kaempferol 3-O-galactoside, kaempferol 3-O-glucoside, rossoliside (7-methyl-1,4,5-trihydroxynaphthalene 4-O-β-glucopyranoside) | 2 | Budzianowski, 1995; Budzianowski et al., 1993 |
| Drosera spatulata | Leaf rosettes | Triterpene 3-O-acetylaleuritolic acid (3-O-AAA) | 2 | Toton et al., 2020 |
| Drosophyllaceae | ||||
| Drosophyllum lusitanicum | Shoots, callus | 5-hydroxy-4-methoxy-2-naphthalenecarboxylic acid 5-O-β-glucoside (drosophylloside) and 5-hydroxy-4-methoxy-2-naphthalenecarboxylic acid methyl ester; 5-hydroxy-4-methoxy-2-naphthalenecarboxylic acid (ancistronaphthoic acid B), hydroplumbagin 4-O-glucoside (plumbaside A), plumbagin, 3-chloroplumbagin, C-glycosylflavones: vitexin, isovitexin, orientin, isoorientin | 2 | Budzianowski et al., 2002 |
| Euphorbiaceae | ||||
| Phyllantus amarus | Non-transformed roots | Lignan (7′-oxocubebin dimethyl ether) | 5 | Sparzak et al., 2015 |
| Fabaceae | ||||
| Cyclopia genistoides | Microshoots and callus | Benzophenones and xanthones | 5 | Kokotkiewicz et al., 2009 |
| Cyclopiasubternata | Cell suspension | Xanthones (mangiferin derivatives), benzophenones, flavones, flavanes and isoflavones | 5 | Kokotkiewicz et al., 2009 |
| Cyclopiasubternata | Suspension cultures in a stirred-tank bioreactor | 7-O-β-glucoside of calycosin, 7-O-glucoside of pseudobaptygenin and 7-O-β-glucoside of formononetin | 5 | Kokotkiewicz, Luczkiewicz, Kowalski, et al., 2013; Kokotkiewicz, Luczkiewicz, Pawlowska, et al., 2013 |
| Genista tinctoria | Callus | Isoflavonoids (14 metabolites) | 5 | Luczkiewicz et al., 2014; Łuczkiewicz & Głód, 2003 |
| Genista tinctoria | Shoot/root co cultures | Derivatives of genistin and daidzin | 5 | Łuczkiewicz & Głód, 2005; Łuczkiewicz & Kokotkiewicz, 2005a |
| Genista tinctoria | Transformed roots | Isoliquiritigenin | 5 | Łuczkiewicz & Kokotkiewicz, 2005b |
| Genista tinctoria | Cell suspension | Isoflavones with genistin as a main component | 5 | Luczkiewicz & Kokotkiewicz, 2012 |
| Lamiaceae | ||||
| Agastache rugosa | Shoot cultures | Methyl chavicol (estragole), menthone, isomenthone, limonene, pulegone, α-pinene, piperitone, β-caryophyllene, α-humulene, germacrene D | 8 | Zielińska et al., 2011, 2017 |
| Agastache rugosa | Shoot cultures | Rosmarinic acid, rosmarinic acid methyl ester, cryptochlorogenic acid, feruoylquinic acid, two ferulic acid glucoside isomers, apigenin derivative | 8 | Zielińska et al., 2019 |
| Moluccella laevis | Callus and shoot cultures | Caffeic acid, ferulic acid, chlorogenic acid, rosmarinic, cyanidin 3-O-galactoside, cyanidin 3-O-malonylglucoside | 8 | Zielińska et al., 2020 |
| Salvia austriaca | Hairy roots | Taxodione, 15-deoxy-fuerstione, royleanone, taxodone and 7-2′-oxohexyl-taxodione | 6 | Kuźma, Wysokińska, Różalski, Krajewska, & Kisiel, 2012 |
| Salvia sclarea | Hairy roots | Salvipisone, ferruginol, aethiopinone, 1-oxoaethiopinone, ursolic acid, oleanolic acid, 2α,3α-dihydroxy-urs-12-en-28-oic acid, 2α,3α,24-trihydroxy-urs-12-en-28-oic acid, β-sitosterol, stigmasterol, campesterol | 6 | Kuźma et al., 2006 |
| Scutelaria barbata | Non-transformed roots | Flavone glycosides (wogonoside, baicalin), phenylethanoid – acteoside | 5 | Wilczańska-Barska et al., 2011, 2012 |
| Scutelaria lateriflora | Hairy roots | Flavone glycosides (wogonoside, baicalin), phenylethanoid – acteoside | 5 | Wilczańska-Barska et al., 2011, 2012 |
| Scutellaria baicalensis | Hairy roots | Flavones – glycosides of: baicalein, wogonin, oroxylin A, chrysin, norwogonin, 5,7,2′-trihydroxyflavone, 5,2′-dihydroxy-6,7,8-trimethoxyflavone and 5,2′,6′-trihydroxy-6,7,8-trimethoxyflavone | 7 | Stojakowska & Malarz, 2000; Stojakowska et al., 2001 |
| Lycopodiaceae | ||||
| Huperzia selago | Sporophytes | Huperzine A | 1 | Szypuła et al., 2005 |
| Huperzia selago | Gamethophytes, prothallus | Huperzine A | 1 | Szypuła et al., 2020 |
| Papaveraceae | ||||
| Chelidonium majus | Callus and shoot cultures | Chelidonine, sanguinatine, chelerythrine, protopine, coptisine, berberine | 8 | Zielinska et al., 2018 |
| Plantaginaceae | ||||
| Plantago lanceolata | Plants, micropropagated plants, callus | Acteoside (verbascoside), plantamajoside, lavandulifolioside, leucosceptoside A, martynoside, desrhamnosylisoacteoside, plantainoside D, desrhamnosylacteoside, lancetoside, i.e., 2-(4-hydroxyphenyl)ethyl O-β-d-glucopyranosyl-(1→3)-[trans- or cis-p-coumaroyl-(→4)]-β-d-glucopyranoside | 2 | Budzianowska et al., 2004 |
| Plantago media | Micropropagated plants (shoots and roots) | Acteoside, plantamajoside | 2 | Budzianowska et al., 2019 |
| Primulaceae | ||||
| Primula veris | Plantlets | 3′-hydroxy-4′,5′-dimethoxyflavone, 3′-methoxy-4′,5′-methylenedioxyflavone, 3′,4′-dimethoxyflavone, 2′,5′-dimethoxyflavone, flavone, 2′-hydroxyflavone, 2′-methoxyflavone, 3′-methoxyflavone, 3′,4′,5′-trimethoxyflavone, 5,6,2′,6′-tetramethoxyflavone (zapotin) | 2 | Budzianowski et al., 2005 |
| Rosaceae | ||||
| Chaenomeles japonica | Micropropagated plants (shoots and roots), callus, cell suspension | Pentacyclic triterpenoids (ursolic acid, oleanolic acid, betulinic acid), polyphenols [chlorogenic acid, (−)-epicatechin, quercetin-3-hexoside] | 2 | Kikowska et al., 2018, 2019 |
| Rutaceae | ||||
| Ruta graveolens | Shoot cultures | Psoralen, bergapten, xanthotoxin, isopimpinellin | 3 | Ekiert, 2004; Ekiert et al., 2001; Ekiert & Czygan, 2005, 2007; Ekiert & Gomółka, 1999; Ekiert & Kisiel, 1997 |
| Ruta graveolens | Shoots co-culture with Ammi majus hairy roots | Xanthotoxine | 4 | Sidwa-Gorycka et al., 2003 |
| Ruta graveolens ssp. divaricata | Shoot-differentiating callus cultures | Bergapten, xanthotoxin, isopimpinellin | 3 | Ekiert, 2004; Ekiert et al., 2005; Ekiert & Czygan, 2005, 2007 |
| Schisandraceae | ||||
| Schisandra. chinensis, Schisandra chinensis ‘Sadova’ | Agar, stationary liquid agitated and bioreactor microshoot cultures | Dibenzocyclooctadiene lignans (e.g., deoxyschisandrin, schisandrin and gomisin A) | 3 | Szopa & Ekiert, 2011, 2014 |
| Schisandra henryi | Agar microshoot and callus cultures | Dibenzocyclooctadiene lignans (schisandrin, gomisin G, schisantherin A and B, deoxyschisandrin and schisandrin C), dibenzylbutane lignans (henricine B), and aryltetralin lignans (wulignan A1 and A2, epiwulignan A1, enshicine, epienshicine and dimethylwulignan A1) | 3 | Jafernik et al., 2020 |
| Schisandra rubriflora | Agar microshoot cultures (♂ and ♀ lines) | Dibenzocyclooctadiene lignans (schisantherin A and B, schisandrin, schisandrin C, gomisin A, D, G, J, N, O, 6-O-benzoylgomisin O, schisandrin A, rubrisandrin A, epigomisin O, schisanhenol, interiotherin C, angeloylgomisin H and O), aryltetralin lignan (wulignan A1), dibenzylbutane lignans (pregomisin, mesodihydroguaiaretic acid), tetrahydrofuran lignan (fragransin A2), and neolignans (licarin A and B) | 3 | Szopa, Dziurka, et al., 2018 |
| Taxaceae | ||||
| Taxus ×media | Hairy roots | Paclitaxel | 1 | Furmanowa & Sykłowska-Baranek, 2000 |
| Taxus ×media | ATMA hairy root line carrying taxadiene synthase transgene | Paclitaxel, baccatin III | 1 | Sykłowska-Baranek, Grech-Baran, et al., 2015; Sykłowska-Baranek, Pilarek, et al., 2015 |
| Taxus ×media | ATM hairy root line carrying taxadiene synthase transgene | Paclitaxel, baccatin III | 1 | Sykłowska-Baranek, Grech-Baran, et al., 2015; Sykłowska-Baranek, Pilarek, et al., 2015 |
| Taxus×media | KT hairy root line | Paclitaxel | 1 | Sykłowska-Baranek et al., 2019 |
| Taxus ×media | ATMA hairy root line carrying taxadiene synthase transgene | Paclitaxel, baccatin III | 1 | Sykłowska-Baranek et al., 2019 |
* 1 – Department of Pharmaceutical Biology and Medicinal Plants Biotechnology, Faculty of Pharmacy, Medical University of Warsaw; 2 – Department of Pharmaceutical Botany and Plant Biotechnology, Poznań University of Medical Sciences; 3 – Department of Pharmaceutical Botany, Jagiellonian University, Collegium Medicum; 4 – Department of Biotechnology, Intercollegiate Faculty of Biotechnology UG & MUG, University of Gdańsk; 5 – Department of Pharmacognosy with Medicinal Plant Garden, Faculty of Pharmacy, Medical University of Gdańsk; 6 – Department of Biology and Pharmaceutical Botany, Medical University of Łódź; 7 – Maj Institute of Pharmacology, Polish Academy of Sciences; 8 – Department of Pharmaceutical Biotechnology and Department of Pharmaceutical Biology and Botany, Faculty of Pharmacy, Wroclaw Medical University.
The History of In Vitro Cultures in Polish Scientific Centers With the Pharmaceutical Profile
Scientific research on using plant in vitro cultures to the study of medicinal plants was initiated at the Pharmaceutical Faculties in Warsaw, Poznań, Kraków, Łódź, and Gdańsk in the 1970s. Prof. Mirosława Furmanowa (1927–2014) was the first in Poland to introduce this field of research to the Department of Pharmaceutical Biology and Botany at the Faculty of Pharmacy of the Medical Academy in Warsaw (presently the Department of Pharmaceutical Biology and Medicinal Plant Biotechnology, Faculty of Pharmacy, Medical University of Warsaw). The initial achievements of the Department in in vitro secondary metabolite biosynthesis were connected with the tissue cultures of plant species alien to Polish flora. Liriodenine, an antifungal alkaloid, was found in Liriodendron tulipifera L. callus cultures. Tingenone, a cytotoxic triterpene, was detected in the callus tissue of the tropical plant Maytenus wallichiana R. et B. In addition, diosgenin, a steroidal sapogenin, was found in Dioscorea deltoidea Wall. and Trigonella foenum-graecum L. callus and cell suspension cultures.
Dr. Jadwiga Halina Supniewska (1913–1984) initiated research on secondary metabolism in Solanum laciniatum Ait., Ammi visnaga Lam., Strophanthus intermedius Pax., and Tecoma stans Juss. callus and cell suspension cultures in the Plant Tissue Culture Laboratory at the Department of Phytochemistry, Maj Institute of Pharmacology, PAS, Kraków, in the late 1960s.
Prof. Stanisław Kohlmünzer, the Head of the Department of Pharmaceutical Botany, Faculty of Pharmacy, Medical Academy in Kraków (since 1993, Faculty of Pharmacy, Collegium Medicum of Jagiellonian University), initated the idea for establishment of a laboratory for plant biotechnology in the 1970s. Dr. Elżbieta Cyunel and Prof. Halina Ekiert started studies on secondary metabolite production in in vitro cultures. The first object of interest was in vitro cultures of Scrophularia scopoli Hoppe, Phytolacca sp., Basella alba L., and Nicotiana tabacum var. Samsun. Some investigations were performed in cooperation with Dr. Barbara Dohnal from the Maj Institute of Pharmacology, PAS [in vitro cultures of Holarrhena antidysenterica (Roxb.) Wall., Catharanthus roseus (L.) G. Don.] and Phytolacca sp. (Ekiert et al., 2017).
The first experiments on in vitro plant cultures in the Department of Pharmacognosy, Medical University of Gdańsk, including phytochemical analysis of in vitro cultures of Rudbeckia hirta L., Vaccinium corymbosum ‘Bluecrop’ and Caryopteris incana (Thunb.) Miq., were performed in early 90s under the mentorship of Prof. Wojciech Cisowski.
Department of Plant Protection and Biotechnology (later on Department of Biotechnology) IFB UG and MUG headed by Prof. Ewa Łojkowska was the second institution in Gdańsk to start studying in vitro plant cultures in 1994, including in vitro micropropagation of endangered plants such as Drosera sp. and Dionaea muscipula and several species of Polish orchids: Cypripedium calceolus L., Dactylorhiza majalis, Epipactis atrorubens, Epipactis palustris, and Orchis morio. Later studies focused on coumarin and furanocoumarin elicitation from in vitro Ammi majus and Ruta graveolens cultures. Studies have focused on the induction of secondary metabolite production from coumarins and naphthoquinones with antibacterial, antioxidative, and cytotoxic activities.
In 1979, at the Department of Pharmaceutical Botany of the Medical Academy in Poznań (currently Poznań University of Medical Sciences), Prof. Lutosława Skrzypczak, the head of this unit, Dr. Maria Wesołowska, and Prof. Barbara Thiem, initiated research in in vitro plant culture in relation to phytochemical analysis, such as the search for new biotechnological sources of secondary metabolites with a biological effect, including Fagopyrum esculentum Moench., Plantago lanceolata L. and in vitro androgenetic cultures of Hyoscyamus niger L. and Hyoscyamus albus L.
. In Vitro Plant Culture Studies for Specific Bioactive Compounds
Paclitaxel in Taxus ×media Hairy Root Cultures
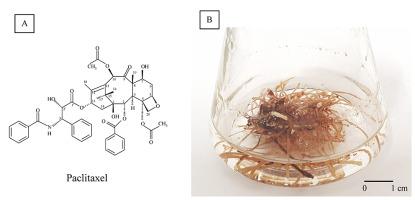
Paclitaxel (Figure 1A), originally named Taxol, is a well-established antineoplastic drug (Yared & Tkaczuk, 2012). The bark of various Taxus species, evergreen, low-growing plants of the Taxaceae family, is the natural source of paclitaxel, but other methods for their production have been elaborated because of its low concentration [0.02% dry weight (DW)] and the necessity of whole plant destruction.
The medical importance of paclitaxel has prompted efforts to develop highly productive Taxus spp. in vitro. Hairy root cultures of Taxus ×media (Figure 1B) were established via yew transformation with Agrobacterium rhizogenes LBA 9402 (Furmanowa & Sykłowska-Baranek, 2000). The paclitaxel content of these roots was 69 µg/g DW, which increased to 210 µg/g DW by elicitation with methyl jasmonate (MJ). Furthermore, combined l-phenylalanine (PHEN) and MJ treatment improved the yield up to 319.7 µg/g DW (Syklowska-Baranek et al., 2009). Two new hairy root lines carrying the taxadiene synthase transgene, ATMA and ATM, were also obtained. Elicitation and precursor feeding strategy (MJ, PHEN) improved paclitaxel yield in ATMA and ATM root line cultures to 10.78 and 1.63 mg/L, respectively (Sykłowska-Baranek, Grech-Baran, et al., 2015). Next, the ATMA root line was cultivated in hybrid two-phase perfluorodecalin-supported cultures (Sykłowska-Baranek, Pilarek, et al., 2015). The degassed perfluorodecalin and MJ increased paclitaxel production to 1,440.08 µg/g DW. Further, the single elicitation with MJ, PHEN, and sodium nitroprusside has been the most efficient in stimulating paclitaxel biosynthesis (2,473.29 µg/g DW) when combined with degassed perfluorodecalin (Sykłowska-Baranek et al., 2019). Significant progress has been made in the development of biotechnological approaches for the production of paclitaxel and other taxanes via Taxus spp. cell cultures on a commercial scale. Nevertheless, the observed increase in demand for taxanes for therapeutic purposes creates a space for developing new stable and efficient culture systems for their production.
Huperzine A in Various In Vitro Huperzia selago Cultures
Among the alkaloids identified only in Lycopodiaceae s. l., quinolizine, pyridine, and α-pyridone-type alkaloids are potent and reversible acetylcholinesterase inhibitors (Ma & Gang, 2004; Muszyński, 1955; Szypuła & Pietrosiuk, 2021). Moreover, huperzine A (HupA, selagine) and huperzine B (HupB) easily cross the blood–brain barrier, are highly acetylcholinesterase-specific inhibitors, and inhibit butyrylcholinesterase at high concentrations (Ferreira et al., 2016; Szypuła & Pietrosiuk, 2021).
With the growing interest in the therapeutic applications of HupA, the demand for club mosses as a source of HupA and other alkaloids is increasing rapidly, and studies on in vitro cultures of Lycopod are gaining importance (Szypuła & Pietrosiuk, 2021).
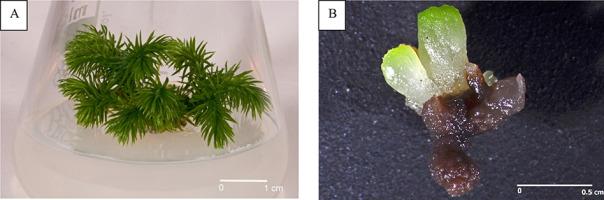
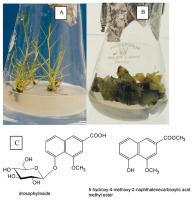
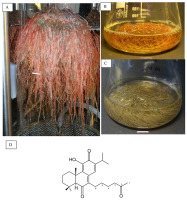
Till date, procedures for establishing and maintaining in vitro cultures of Huperzia selago sporophytes (Figure 2A) and gametophytes (Figure 2B) have been developed using various starting materials, including shoot fragments, vegetative propagules, somatic embryos, and spores (Szypuła & Pietrosiuk, 2021; Szypuła et al., 2005, 2013, 2020).
The first studies on HupA biosynthesis were conducted by Szypuła et al. (2005, 2011, 2013). The establishment of H. selago sporophyte in vitro cultures initiated from shoot explants and vegetative propagules (bulbils) allowed approximately tenfold reduced developmental stages with the omission of the gametophyte stage, thus increasing the sporophyte mass tenfold within 6 months. The sporophytes regenerating from somatic embryos produced the highest reported Lycopod yield of HupA till date (3.33 mg/g DW).
Using spores from sporophytes procured from natural populations is an effective method for establishing in vitro axenic cultures of H. selago gametophytes (Szypuła et al., 2020). Prothalli were obtained after 7–18 months, and optimal results were achieved on the medium originally developed by Whittier and Storchova (2007) and on Moore medium as modified by Freeberg and Wetmore (1957). Using H. selago gametophytes can increase HupA biosynthesis by approximately 42% than sporophyte culture, with its yield being 35-fold higher than that of Hydrangea serrata, used in the pharmaceutical industry (Szypuła et al., 2020). HupA content in H. selago prothallus is 0.74–4.73 mg/g DW, which is the maximum reported for club mosses (Szypuła et al., 2020).
Naphthoquinone Derivatives From In Vitro Cynoglossum columnae and Rindera graeca roots
Shikonin derivatives present multidirectional pharmacological activities including wound healing, anti-inflammatory, antimicrobial, and anticancer properties (Papageorgiou et al., 1999, 2006, 2008). In addition, the simultaneous use of shikonin derivatives and classical cancer therapies has a promising synergistic effect (He et al., 2016; Jeziorek et al., 2019; Wang et al., 2014; Zhao et al., 2015).
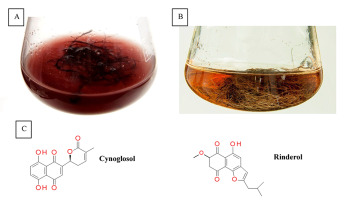
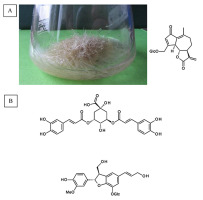
Naphthoquinone pigments with chemotaxonomic and pharmaceutical significance have been derived from Cynoglossum columnae Ten. in vitro root cultures (Figure 3A). The root extracts and postculture media were subjected to detailed chromatographic analysis, which allowed to isolate two novel naphthoquinone derivatives, rinderol and cynoglosol (Jeziorek et al., 2019) (Figure 3C). The cytotoxicity of both compounds was tested against HL-60, HeLa, and HCT-116 cancer cell lines. Rinderol showed the highest activity against HL-60 cells (IC50 = 2 µg/mL), while cynoglosol was selectively active against HL-60 cells (IC50 = 4.3 µg/mL). Rinderol also demonstrated an antimicrobial activity against Staphylococcus epidermidis ATCC 12228 (MIC = 3.91 µg/mL) and Staphylococcus epidermidis MRSE 456 (MIC = 7.81 µg/mL) (Jeziorek et al., 2019).
Rinderol was detected in Rindera graeca (A. DC.) Bois. & Heldr. hairy root cultures (Figure 3B) obtained via Agrobacterium rhizogenes ATCC 15834 infection (Graikou et al., 2021; Pietrosiuk et al., 2006; Sykłowska-Baranek et al., 2008, 2012). The isolated rinderol exhibited cytotoxic activity against two human lung cancer cell lines NSCLC-N6-L16 (IC50 = 1.2 µg/mL) and A549 (IC50 = 0.9 µg/mL).
Phenylethanoid glycosides in Plantago media and In Vitro Plantago lanceolata Cultures
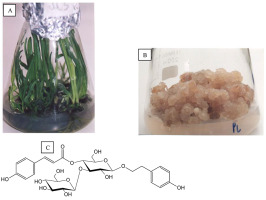
The in vitro Plantago lanceolata L. cultures involved shoots developed on leaves (Figure 4A), root explants, and two root- and callus-leaf-derived lines (Figure 4B). A total of 16 phenylethanoid glycosides were isolated, including those new to the species and a novel natural product, lancetoside (Figure 4C). Plantamajoside and acteoside are the main constituents of calli and leaves, respectively (Budzianowska et al., 2004).
Plantago media L. was cultured on Murashige and Skoog (MS) (1962) medium and the best medium was supplemented with 2.0 mg/L 6-benzylzdeninpurine (BA) and 0.1 mg/L indolyl-3-acetic acid (IAA). A TLC video densitometric assay showed high acetoside and plantamajoside content in shoots and roots, respectively (Budzianowska et al., 2019).
Phenolic Acids and Triterpenoid Saponins in In Vitro Eryngium planum Cultures
Eryngium planum L. from the Apiaceae family, a species native to Poland, contains a rich set of secondary metabolites (Kikowska, 2014). The biomass for phytochemical analysis has been obtained using clonal propagation (Figure 5A). The total content of selected phenolic acids (RA, chlorogenic acid, caffeic acid) as well as that of triterpenoid saponins was higher in micropropagated plantlet shoots and roots than those in analogous soil-grown plant organs (Kikowska et al., 2019; Thiem et al., 2013).
The influence of elicitor type and elicitation duration on phenolic acid accumulation in E. planumshoots has been studied (Figure 5B). MJ solution, high concentration sucrose (S) solution, and elicitation with yeast extract (YE) increased the RA content in shoots by up to 5.5, 4.7, and 2.3 times, respectively (Kikowska et al., 2015).
Figure 5
(A) Shoot multiplication as a step of Eryngium planum micropropagation. (B) Agitated E. planum shoots.

Bioactive compounds can also be produced in E. planum callus and cell suspension cultures (Figure 6). An MJ-treated suspension increased the total phenolic acid content by 1.65–2.08-fold (Kikowska et al., 2012). Adding high concentration S or MJ solution to the medium enhanced saponin accumulation (up to 1.7 and 1.2 times, respectively). Therefore, elicitation may be effective for enhancing phenolic acid and triterpenoid saponin accumulation in flat sea-holly cultures (Kikowska et al., 2019).
We have previously isolated and characterized triterpenoid saponins from E. planum roots, which quantitatively comprised different in vitro E. planum cultures (Kikowska et al., 2019; Kowalczyk et al., 2014).
Eryngium planum shows amoebicidal activity against pathogenic Acanthamoeba castellanii (Derda et al., 2013), neuroprotective activity (Ożarowski et al., 2015), apoptotic activity (Kikowska, 2014), and antimicrobial activity (Kikowska et al., 2016).
Pentacyclic Triterpenoids in Various In Vitro Chaenomeles japonica Cultures
Homogenous and uniformly stabilized callus and cell suspension cultures (Figure 7A,B) of Chaenomeles japonica (Thunb.) Lindl. ex Spach (Rosaceae), a dwarf shrub used in traditional medicine in the Far East, have been established (Kikowska et al., 2019).
Microscopic observations of fibroblast viability, morphology, and proliferation ratio proved that the callus extract significantly affected fibroblasts. Pentacyclic triterpenoid-rich Ch. japonica callus extract may be a potential source of cosmetic ingredients (Kikowska et al., 2018).
Rossoliside, Plumbaside A, and Related Compounds in In Vitro Carnivorous Species Cultures
The isolated phenolic glycosides rossoliside, new for Drosera spatulata Labill and known for Drosera rotundifolia L. (Droseraceae), and plumbaside A from Drosera intermedia L., are easily hydrolyzed and oxidized with air oxygen to 7-methyljuglone (ramentaceone) and plumbagin, respectively (Budzianowski, 1995, 1996). Moreover, rossoliside is oxidized to an unknown 2-methylnaphthazarin 5-O-glucoside (Budzianowski, 1997). Plumbaside A has also been isolated from in vitro cultures of (i) Dionaea muscipula Ellis (Kukułczanka & Budzianowski, 2002), which yields other phenolics as well (Pakulski & Budzianowski, 1996a, 1996b), (ii) Drosophyllum lusitanicum Link (Drosophyllaceae), together with two new naphthalene derivatives (Figure 8) and other phenolics (Budzianowski et al., 2002), and (iii) Drosera gigantea Lindley with droserone and hydroxydroserone 5-O-glucosides, which contribute to the maroon color of this plant (Budzianowski, 2000).
The triterpene 3-O-acetylaleuritolic acid, a novel compound isolated from D. spatulata leaf rosettes (Figure 9), inhibits HeLa, HT-29, and MCF7 cell growth and migration (Toton et al., 2020).
In turn, in vitro Byblis liniflora Salisb (Byblidaceae) cultures yielded four new phenylethanoid glycosides. The presence of acteoside supports the placement of Byblidaceae in the order Scrophulariales and subclass Asteridae (Schlauer et al., 1994).
Lipophilic Flavones in Primula veris Shoot Cultures
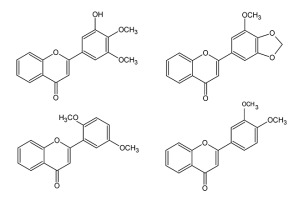
The in vitro propagated Primula veris L. (Primulaceae) contains 10 lipophilic flavones, similar to those isolated from field-cultivated plants, including new compounds (Figure 10) (Budzianowski et al., 2005). Among them, zapotin has been investigated for the mechanism of anticancer activities (Toton et al., 2012, 2016).
Linear Furanocoumarins in Various In Vitro Ammi majus, Pastinaca sativa, Ruta graveolens, and Ruta graveolens ssp. divaricata Cultures
Linear furanocoumarins (LF) are used in photochemotherapy for skin diseases characterized by distorted pigmentation and excessive cell proliferation. They can also be used in cardiology and neurology (Bethea et al., 1999; Bohuslavizki et al., 1994; Ekiert, 2004; Härmälä et al., 1992; Matern, 1999; Wolf, 2016). The most important LF used in therapy include bergapten, xanthotoxin, isopimpinellin, and imperatorin. Umbelliferone is a biogenic precursor of these compounds. Ammi majus L. and Pastinaca sativa L. fruits are rich LF sources for pharmaceutical applications.
The best conditions for LF production (113.83 mg/100 g DW) in the established Ammi majus callus cultures were LS variant (10 mg/L) 6-benzylaminopurine (BAP) and 10 mg/L 2,4-D), and white light (900 lx). The maximum isopimpinellin and imperatorin contents were equal to or exceeded those in the parent plant fruits. These investigations were among the first biotechnological studies from a pharmaceutical perspective in Poland (Ekiert, 1993).
The next stage of research involved the optimization of callus culture conditions for Ammi majus (cultures of other origin) and Pastinaca sativa. The total LF and umbelliferone content in Ammi majus cultures on 12 agar LS medium variants was 40.95–871.00 mg/100 g DW. The LS variant with 0.1 mg/L BAP and 0.1 mg/L 1-naphthylacetic acid (NAA) was the best “production” medium. High imperatorin, xanthotoxin, and umbelliferone levels have also been reported (Ekiert, 2004; Ekiert & Gomółka, 2000a). In contrast, isopimpinellin (90.20–238.90 mg/100 g DW) was the main metabolite accumulated in Pastinaca sativa callus cultivated on 10 LS variants. The total LF and umbelliferone content was 115.70–408.50 mg/100 g DW (Ekiert, 2004; Ekiert & Gomółka, 2000b).
In Western Europe, Ruta graveolens L. has been proposed as a rich LF source in pharmacology and cosmetology. In the established Ruta graveolens stationary liquid shoot cultures the ability to produce psoralen, bergapten, xanthotoxin, and isopimpinellin was documented (Figure 11A) (Ekiert & Kisiel, 1997). In contrast to the cultures under UV-C light or in darkness, the shoots cultured under white light (900 lx) produced equal or higher amounts of LF (1,022.2 mg/100 g DW) than plants growing in different habitats (575.0–1,033.0 mg/100 g DW) (Ekiert & Gomółka, 1999). Moreover, Ruta graveolens shoot cultures in 12 LS medium variants have also been tested. The total LF ranged between 254.2 and 939.7 mg/100 g DW, with xanthotoxin being the main metabolite. The media with 0.1 mg/L BAP and NAA and 2 mg/L BAP and NAA were the best “production” media. Thus, these LS variants were chosen for studying the dynamics of LF accumulation in stationary liquid and agitated cultures (42 days growth cycles) of Ruta graveolens and Ruta graveolens ssp. divaricata (the culture obtained in the framework of cooperation with the Institute of Natural Sciences of the University in Würzburg).
The total LF and umbelliferone content in stationary cultures of rue and its subspecies increased 2,3-fold and 2,3-fold over 48 days and reached 966.0 and 283.4 mg/100 g DW on twenty-eighth and thirty-fifth culture day, respectively (Ekiert, 2004; Ekiert et al., 2001, 2005; Ekiert & Czygan, 2005, 2007). Cultures of both plants produced high bergapten and xanthotoxin amounts. The total LF content in agitated cultures of both plants increased by 4.8- and 2.0-fold over 48 days and reached 580.8 and 64.0 mg/100 g DW on forty-second and thirty-fiveth culture day, respectively (Figure 11B). Xanthotoxin and bergapten were the main metabolites in both plant cultures.
In addition, studies on LF biogenesis have been conducted in cooperation with the Institute of Pharmaceutical Biology, University in Marburg/am Lahn, which resulted with isolation and characterization of bergaptol O-methyltransferase from elicited in vitro Ammi majus cultures (of yet another origin). The isolated enzyme participates in bergapten (5-methoxypsoralen) biosynthesis from bergaptol (5-hydroxypsoralen) (Hehmann et al., 2004).
All presented results documented that in the investigated in vitro culture systems, the production of LF could be high and that biotechnological strategies could develop problems with imports and/or with obtaining high-quality plant material in Europe from in vivo conditions. The scale-up of maintaining in vitro cultures (in large-scale laboratory installations) is recommended in future investigations.
Dibenzocyclooctadiene Lignans in Various In Vitro Schisandra sp. Cultures
Dibenzocyclooctadiene lignans constitute the main secondary metabolites specific to the Schisandra genus (Szopa, Ekiert, & Ekiert, 2017). Schisandra chinensis Turcz. Baill. is the best-known of these species (World Health Organization, 2007, pp. 296–313). These compounds exhibit hepatoprotective, antioxidant, and anti-inflammatory activities (Szopa, Barnaś, & Ekiert, 2019; Szopa, Ekiert, & Ekiert, 2017).
Szopa, Klimek, and Ekiert (2016) aimed to investigate lignan production in in vitro Schisandra genus cultures using different comprehensive and globally innovative biotechnological methods. Experiments have been conducted using Schisandra chinensis, Schisandra chinensis ‘Sadova’, Schisandra henryi C. B. Clarke, and Schisandra rubriflora (Franch.) Rehd. et Wils cultures (Figure 12).
Optimization of S. chinensis agar microshoot culture conditions increased deoxyschisandrin, schisandrin, and gomisin A levels. The maximum total tested lignan content (486.78 mg/100 g DW) in microshoot agar cultures is 1.3- and 3.8-fold higher than that in the parent plant fruits and leaves, respectively (Szopa & Ekiert, 2011, 2014).
The next step of the research involved the optimization of lignan production in different bioreactors (balloon, spray, and column with shoot immobilization system) and temporary immersion systems RITA and PlantForm. Stationary liquid, agitated, and agar cultures have also been studied. The maximum contents of 14 tested lignans in agar, agitated, and PlantForm bioreactor cultures are 237.86, 195.15, and 546.98 mg/100 g DW, respectively (Szopa, Kokotkiewicz, et al., 2016, 2017, 2019).
These studies were extended by the successful establishment of microshoot cultures of the high-yield cultivar S. chinensis ‘Sadova’. The maximum total contents of lignans were also high, reaching 574.42, 375.11, and 313.51 mg/100 g DW in agar, agitated, and PlantForm bioreactor cultures, respectively (Szopa, Klimek-Szczykutowicz, et al., 2018; Szopa, Kokotkiewicz, et al., 2019).
The presence of dibenzocyclooctadiene, dibenzylbutane, and aryltetralin lignans has been confirmed in S. henryi microshoot and callus solid cultures. The total amount of the detected lignans is 873.71 mg/100 g DW. The main compounds are schisantherin A and B (Jafernik et al., 2020).
Biotechnological studies on S. rubriflora involved the ♂ and ♀ lines of microshoot cultures. The presence of dibenzocyclooctadiene, aryltetralin, dibenzylbutane, tetrahydrofuran, and neolignans has been confirmed in the culture extracts. The maximum total content for the detected lignans is 250.92 and 220.70 mg/100 g DW for the ♂ and ♀ lines, respectively. The main compounds are schisantherin B, angeloylgomisin H, and O (Szopa, Dziurka, et al., 2018).
All the presented results document the high biosynthetic potential of cells from in vitro cultures of different Schisandra species and cultivars. The high lignin production could be a valuable alternative for the import of Schisandra fruit from China.
Naphthoquinones in Various In Vitro Drosera and Dionaea Plant Cultures
Plants from the Drosera and Dionaea genera are valuable sources of biologically active naphthoquinones (NQs), with the most pharmacologically significant including plumbagin (PL), ramentaceone (RAM), and 3-chloroplumbagin (3ChPL). These plants are micropropagated through adventitious shoot regeneration from leaf explants and shoot tips (Kawiak et al., 2003). Optimal adventitious Drosera aliciae shoot regeneration was obtained from leaf explants grown on 1/2 MS medium supplemented with 0.4 µM 6-benzyladenine. These growth conditions yielded the highest micropropagation coefficient and increased survival rate by 42%. The genetic fidelity of the shoots, assessed with RAPD, confirmed the genetic homogeneity of the regenerants (Kawiak et al., 2011). Elicitation was employed to increase the secondary metabolite content in Drosera and Dionaea cultures, and was performed by the application of biotic and abiotic elicitors. Biotic elicitation of Drosera and Dionaea cultures with Agrobacterium rhizogenes lysates increased PL content by 2.6-fold from 1.1 mg/g fresh weight (FW) in Dionaea muscipula and RAM content by 1.9-fold from 1.2 mg/g FW in Drosera capensis (Krolicka et al., 2008).
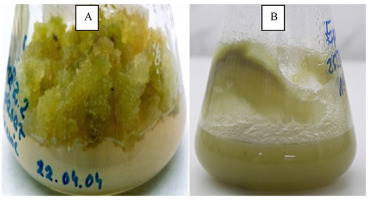
Dionaea muscipula (Figure 13A) and Drosera capensis var. alba (Figure 13B) has been transformed for increasing NQ production. Dionaea muscipula has been successfully transformed using two wild-type Rhizobium rhizogenes strains to form teratoma (transformed shoot) cultures without hairy roots.
Figure 13
Transformed tissue (teratomas) of carnoivorous plants (A) Dionaea muscipula and (B) Drosera capensis var. alba.

HPLC analysis revealed an increase in secondary metabolite content, with a 1.7-fold increase in PL content in transformed tissue (106 mg/g DW) (Makowski et al., 2021). Drosera capensis var. alba transformation formed teratomas without hairy root formation. The growth index increased 3.7-fold and the RAM level in the transformed teratomas increased by 1.6-fold to 410 µg/g DW (Krolicka et al., 2010).
The biological activity of the NQs derived form in vitro cultured plants have been evaluated. RAM isolated from in vitro D. aliciae cultures displayed antibacterial activity, with the highest effects on Staphylococcus aureus. Both the susceptible and antibiotic-resistant strains were sensitive to RAM (MBC 0.05 mg/mL). The mode of RAM activity toward bacterial cells was determined as DNA and RNA synthesis inhibition (Krolicka et al., 2009). RAM also displays cytotoxic activity against human cancer cells. The highest activity of RAM was exerted toward leukemic cells HL-60 (IC50 = 8.7 µM) and U937 (IC50 = 3.2 µM), with the cytotoxic activity toward U937 cells being similar to that of etoposide (Kawiak et al., 2011). The mechanism of RAM action is associated with ROS-mediated apoptosis induction in HL-60 cells (Kawiak et al., 2012).
PL isolated from Drosera binata has high cytotoxic activity toward a panel of cancer cells, including HL-60 cells (IC50 = 2.7 µM). PL exerts its activity in HL-60 cells by stabilizing the topoisomerase II–DNA complex and inducing DNA cleavage, which triggers apoptosis (Kawiak et al., 2007). PL also modulates the activity of chemotherapeutics, including paclitaxel. Moreover, PL downregulates MAPK/ERK signaling in breast cancer cells, which is upregulated in paclitaxel-resistant cells. PL, through decreasing p-ERK levels, overcomes the resistance of breast cancer cells to paclitaxel (Kawiak, Domachowska, Krolicka, et al., 2019; Kawiak, Domachowska, & Lojkowska, 2019).
The 3ChPL found in D. binata has higher bactericidal efficacy than PL. Specifically, it has high activity against S. aureus (MBC = 8 µg/mL). 3ChPL exerts synergistic activity with silver nanoparticles (AgNPs), which further enhances the antimicrobial activity of 3ChPL. Bacterial cell membrane disruption is supposedly the mechanism underlying the synergistic effects of 3ChPL and AgNPs. Synergistic interactions with AgNPs increased the efficacy of reduced 3ChPL concentrations, at which they are non-toxic to human keratinocytes (Krychowiak et al., 2018). Further studies have shown that AgNPs overcome intrinsic microbial resistance to NQs (Krychowiak-Maśnicka et al., 2021). 3ChPL also has high activity toward cancer cells, particularly breast cancer cells (IC50 = 0.6–2 µM). 3ChPL induces apoptosis in breast cancer cells by downregulating the anti-apoptotic Bcl-2 family protein Mcl-1 (Kawiak, Domachowska, Krolicka, et al., 2019).
Coumarins and Furanocoumarins in Various In Vitro Ammi majus and Ruta graveolens Cultures
Ammi majus and Ruta graveolens are sources of coumarins (umbelliferone) and furanocoumarins (psoralen, xanthotoxin, bergapten, and imperatorin). Ammi majus callus, cell suspension, and hairy root cultures obtained using two agropine R. rhizogenes strains have been established as potential coumarin and furanocoumarin sources. Umbelliferone was detected in all cultures, with the highest level (19 µg/g DW) determined in hairy root cultures (Królicka et al., 2001). Umbelliferone content further increased 96-fold in A. majus callus cultures (9.6 mg% DW) and 1.2-fold in hairy root cultures (2.3 mg% DW) with biotic elicitation (Staniszewska et al., 2003). Further studies used A. majus and R. graveolens to establish a co-culture of these plants for furanocoumarin synthesis in R. graveolens cultures utilizing umbelliferone substrate released by A. majus cultures.
The most efficient co-culture model included A. majus hairy roots and R. graveolens shoots. A 2.5-fold increase in furanocoumarin and xanthotoxine (38.1 mg/g DW) synthesis was reported (Sidwa-Gorycka et al., 2003).
Isoflavonoids in Various In Vitro Genista tinctoria Cultures
At the Department of Pharmacognosy (Medical University of Gdańsk), in vitro Genista tinctoria L. cultures have been obtained as a source of phytoestrogens used in hormone-dependent tumor treatment and mitigate the effects of menopause. Callus cultures of the species [Schenk and Hildebrandt (SH) medium with 2,3,5-triiodobenzoic acid (TIBA) and kinetin] selectively produced 14 isoflavonoids (10,474.23 mg/100 g DW) (Luczkiewicz et al., 2014; Łuczkiewicz & Głód, 2003), without a set of toxic quinolizidine alkaloids. Cultivating the biomass as suspension and after elicitor treatment (MJ and chitosan) obtained 11,138.95 mg isoflavones from 100 g DW (the main metabolite – genistin – 9,414.7 mg/100 g DW), of which 80% was released from the plant matrix into the medium [permeabilization with dimethyl sulfoxide (DMSO)] (Luczkiewicz & Kokotkiewicz, 2012). The suspension may be considered an in vitro plant system for profitable estrogen/mimetic isoflavone production (Figure 14A).
Further research resulted in plant system development as intra-species (shoot/hairy root) G. tinctoria co-cultures (Łuczkiewicz & Kokotkiewicz, 2005a). Liquid plant microshoot monocultures (SH medium with IBA) produce phytoestrogens which belong to the genistein metabolic pathway with relatively low daidzein derivative production (Łuczkiewicz & Głód, 2005). Genista tinctoria hairy roots produce only isoliquiritigenin, which is a direct daidzein precursor, not found in the intact plant (Łuczkiewicz & Kokotkiewicz, 2005b). Both biomass types were combined in a co-culture system (basket-bubble bioreactor, SH medium with IBA). Isoliquiritigenin is translocated from the roots to the medium only after supplementing the plant system with abscisic acid, allowing its bioconversion to daidzein and daidzin in the shoots (Figure 14B). The system synthesized genistein derivatives at the level of microshoot monocultures, “enriched” with the additional daidzin and daidzein concentration. Productivity and specific productivity of the system in terms of genistin (1,381 mg/L and 34.53 mg/day, respectively) and daidzin (434.90 mg/L and 8.0 mg/day, respectively) accumulation indicates that it may be used for the industrial production and bioconversion of selected phytoestrogens (Łuczkiewicz & Głód, 2005).
Xanthones and Polyphenolic Compounds in Various In Vitro Cyclopia subternata and Cyclopia genistoides Cultures
Microshoots and calli from selected African honeybush plants (Cyclopia genus) have been used to develop in vitro plant systems designed to produce xanthones and polyphenolic compounds with antioxidant, anti-inflammatory, and estrogen/mimetic activities (Kokotkiewicz et al., 2009). Cyclopia subternata suspension selectively accumulates three isoflavones absent in the intact plant, while Cyclopia genistoides microshoots synthesize benzophenones and xanthones identical to those found in the intact plant (Kokotkiewicz et al., 2009). After increasing isoflavone and selected xanthone and benzophenone accumulation in these cultures (retrobiosynthetic strategies, elicitation, and immobilization), both the suspension and microshoots were upgraded for growth in prototype bioreactors designed at the Department of Pharmacognosy, Medical University of Gdańsk (Kokotkiewicz, Luczkiewicz, Kowalski, et al., 2013; Kokotkiewicz, Luczkiewicz, Pawlowska, et al., 2013). Cyclopia subternata suspension cultures were grown in a stirred-tank bioreactor (MS medium with 4-CPPU, 2,4,5-T, and 5% v/v coconut water) and synthesized 7-O-β-glucoside in calycosin, 7-O-glucoside of pseudobaptygenin, and 7-O-β-glucoside of formononetin (their respective productivities were 0.96, 0.44, and 0.22 mg/L/day, respectively) (Figure 15A) (Kokotkiewicz, Luczkiewicz, Pawlowska, et al., 2013). Cyclopia genistoides microshoots produce high xanthone and benzophenone concentrations (Kokotkiewicz, Luczkiewicz, Kowalski, et al., 2013) and have been used for growth in a temporary immersion bioreactor (SH medium with IBA) (Figure 15B). They produced mangiferin, isomangiferin, hesperidin, 3-C-β-glucoside iriflophenone, and maclurine and the productivity calculated (5.48, 1.58, 0.72, 3.04, and 0.06 mg/L/day, respectively) indicate the potential to use the system for continued production of these metabolites, independent of environmental conditions (Kokotkiewicz, Luczkiewicz, Kowalski, et al., 2013). The compounds isolated from C. genistoides cultures have been screened for pro-apoptotic activity in TNF-α-stimulated synovial cells isolated from rheumatoid arthritis patients. The strongest effect, measured as the apoptotic cell percentage, has been recorded for isomangiferin (75%), followed by iriflophenone-3-β-glucoside (71%), and mangiferin (65%), which is an indication for further biological research into their application in rheumatoid arthritis treatment.
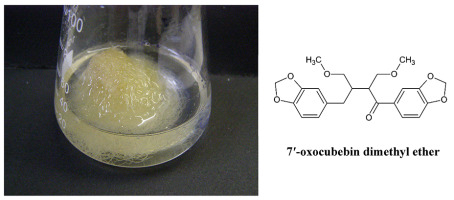
Lignans in Non-Transformed Phyllantus amarus Root Cultures
The roots and aerial parts of many species of the genus Phyllantus are a source of medicinal plant materials, including lignans, which are the main biologically active compounds. In our study, non-transformed Phyllantus amarus root cultures (Figure 16) have been established and cultivated on SH medium with IBA and 1/2 macro and micro salt concentration as a selective source of only one lignan, 7′-oxocubebin dimethyl ether (Figure 16). This is a new compound in the plant kingdom and has strong cytotoxic activity against the HeLa cell line (IC50 = 3.8 µg/mL) (Sparzak et al., 2015).
Flavone Glycosides and Phenylethanoid in Scutellaria sp. Hairy and Non-Transformed Root Cultures
Many species of the genus Scutellaria are medicinal plants that contain biologically active flavones. Their concentration is the highest in Scutellaria baicalensis roots after cultivating for approximately 6 years. Two in vitro root cultures have been established, the non-transformed Scutellaria barbata roots (B5 with IBA and SH with IBA; Figure 17A,B) and Scutellaria lateriflora hairy roots (Figure 18), which mainly accumulates flavone glycosides (Wilczańska-Barska et al., 2011, 2012). The flavone productivity in Scutellaria lateriflora hairy roots (478 mg/L) was 2.5-fold lower than that in S. barbata root culture; however, both cultures accumulated more flavones than S. baicalensis hairy roots. Using Pectobacterium carotovorum suspension, a plant pathogen, changed the production profile and stimulated the selective aglycone-wogonin accumulation in hairy roots (3% DM). Scutellaria lateriflora hairy roots produce can be a rich source of wogonin with antioxidant, anti-inflammatory, and neuroprotective activities as a phytoalexin (Wilczańska-Barska et al., 2012).
Scutellaria baicalensis hairy roots obtained by genetic transformation with Rhizobium rhizogenes LBA 9402 produced up to 10% DW major flavones (baicalein, wogonin, oroxylin A, and their glycosides) when cultured in an optimized nutrient medium supplemented with 5%–7% sucrose (Stojakowska & Malarz, 2000). A detailed analysis of flavone aglycones synthesized in the culture revealed the presence of additional compounds such as chrysin, norwogonin, 5,7,2′-trihydroxyflavone, 5,2′-dihydroxy-6,7,8-trimethoxyflavone, and 5,2′,6′-trihydroxy-6,7,8-trimethoxyflavone (Stojakowska et al., 2001). The results have been cited in several Korean patents aimed at inventing compositions to prevent and treat enteroviral diseases.
Diterpenoids in Salvia sclarea Transformed Root Cultures
Salvia sclarea L. is an aromatic herbaceous plant belonging to the Lamiaceae family that grows naturally in the Mediterranean basin, Southern Europe, and Iran. This species is used commercially in many countries for medicinal and cosmetic purposes. Salvia sclarea plant essential oil has been described in the European Pharmacopoeia (Salviae sclareae aetheroleum).
Under in vitro conditions, Salvia sclarea shoots have been transformed with Rhizobium rhizogenes LBA 9402 strain containing the pRi 1855 plasmid. After bacteria elimination, the clary sage hairy roots were cultured in 300-mL Erlenmeyer flasks containing 80 mL half-strength macro- and microelement liquid sterile growth regulator-free Gamborg medium (1/2 B5) (Figure 19A) (Gamborg et al., 1968) supplemented with 3% sucrose. Genetic transformation in sclarea hairy roots has been confirmed using molecular methods (Kuźma et al., 2006). Hairy root cultures were maintained in a rotary shaker. Roots were cultivated in two lines: light and dark. The biomass of the hairy roots grown in light was 72.8 ± 2.9 and 6.75 ± 0.25 g/L fresh and dry, respectively. Light promoted both biomass and diterpenoid production in S. sclarea hairy roots (Kuźma et al., 2006, 2008, 2009), therefore MJ elicitation of S. sclarea hairy roots in light was studied by adding 62.5–1,250 µM MJ on day 23. The 7-day treatment of the Salvia sclarea hairy roots with 125 µM MJ was the best condition for biomass and secondary metabolite production, particularly in bioreactors (Kuźma et al., 2009) (Figure 19B).
Four diterpenoids, salvipisone, ferruginol, aethiopinone, and 1-oxoaethiopinone, have been isolated from S. sclarea (Kuźma et al., 2006). The further separations isolated ursolic acid, oleanolic acid, 2α,3α-dihydroxy-urs-12-en-28-oic acid, 2α,3α,24-trihydroxy-urs-12-en-28-oic acid, β-sitosterol, stigmasterol, and campesterol (Kuźma et al., 2006). The dark- and light-grown roots biosynthesize the same secondary metabolites, but quantitative HPLC analysis has reported that light promotes diterpenoid biosynthesis (Kuźma et al., 2006).
The antibacterial properties of the diterpenoids isolated from S. sclarea hairy roots were tested. Salvipisone had the highest antibacterial activity, possible due to bacterial adhesion and biofilm formation inhibition (Kuźma et al., 2007). Diterpenoids isolated from S. sclarea hairy roots were also tested for activity against Staphylococcus aureus (MRSA) and Staphylococcus epidermidis (MRSE) strains. Salvipisone and aethiopinon exhibit strong synergistic actions with antibiotics (Walencka et al., 2007). The cytotoxic properties of the diterpenoids isolated from clary sage hairy roots have also been tested against NALM-6, HL-60, and HL-60 ADR cells (Różalski et al., 2006).
Diterpenoids in Transformed Salvia austriaca Root Cultures
Salvia austriaca is an herbaceous plant native to Russia and Eastern Europe (Clebsch & Barner, 2003). The roots of this species can accumulate abietane-type diterpenoids (Nagy et al., 1999). After infecting in vitro cultivated S. austriaca shoots with Rhizobium rhizogenes A4 strain, genetically modified roots were obtained (Kuźma et al., 2011), which were maintained in half-strength Gamborg (1/2 B5) liquid medium supplemented with 3% sucrose in light (Gamborg et al., 1968) (Figure 19C). The fresh and dry biomass of the 30-day-old transformed root culture increased by 11- and 9-fold, respectively (Kuźma et al., 2011). The influence of culture medium type (and the half-strength of micronutrient variants) on growth and diterpene biosynthesis has been estimated (Kuźma et al., 2017). Biomass and diterpene production in shake flask culture has been examined using SH, Gamborg (B5) and woody plant (WPM) media, with both full and half-strength macro- and micronutrient concentrations supplemented with 3% sucrose.
N-hexane S. austriaca hairy root extract has been chromatographically separated (Kuźma et al., 2011) and three abietane-type diterpenoids have been identified, including taxodione, 15-deoxy-fuerstione, and royleanone, for the first time. Subsequently, taxodone and 7-2′-oxohexyl-taxodione has been isolated (Figure 19D) (Kuźma, Wysokińska, Różalski, Krajewska, & Kisiel, 2012). The last compound appears to be a novel compound previously unknown in the plant kingdom.
The cytotoxicity of taxodione, taxodone, 15-deoxyfuerstione, and 7-2′-oxohexyl-taxodione against HL-60, NALM-6, WM-115, and healthy HUVEC has been tested (Kuźma, Wysokińska, Różalski, Krajewska, & Kisiel, 2012). 7-2′-Oxohexyl-taxodione, an unusual diterpenoid, exhibits tenfold stronger cytotoxic activity than the highly active taxodione against all tested neoplastic cell lines. Moreover, taxodione and 7-2′-oxohexyl-taxodione exhibits seven- and eightfold lower cytotoxicity, respectively, against healthy cells (HUVEC) than that against neoplastic cells (Kuźma, Wysokińska, Różalski, Krajewska, & Kisiel, 2012). The antibacterial and antifungal actions of taxodione and 7-2′-oxohexyl-taxodione against the reference strains Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans, and the clinical strains Staphylococcus aureus and Enterococcus spp. have also been examined. 7-2′-Oxohexyl-taxodione demonstrated strong activity against gram-positive bacteria, particularly Staphylococcus aureus (MRSA). This diterpenoid exhibits 4 times stronger activity than taxodione (Kuźma, Wysokińska, Różalski, Budzyńska, et al., 2012).
Sesquiterpenoids and Phenolics in Cichorium intybus and Lactuca spp. Hairy Roots and Undifferentiated Cultures
Lactucin, lactucopicrin, and 8-deoxylactucin are bitter-tasting sesquiterpene lactone (SL) guaianolides, demonstrating several biological activities both in vivo and in vitro (European Medicines Agency, 2013; Gromek et al., 1992). These compounds are secondary metabolites of wild growing and cultivated numerous Lactuca and Cichorium species and are accumulated mainly in the plant roots. Similar to many other SLs, 8-deoxylactucin and lactucopicrin demonstrate anti-inflammatory activity. Moreover, these compounds inhibit α-glucosidase function (Fan et al., 2017). The analgesic activity and sedative property of lactucopicrin are similar to those of ibuprofen as shown in a spontaneous locomotor activity test in mice (Wesołowska et al., 2006). This result was similar to that observed for active Lactuca virosa preparations containing 8-deoxylactucin (Gromek et al., 1992).
Lettuce and chicory are closely related taxonomically and produce similar specialized metabolites, including SLs, coumarins, flavonoids, and hydroxycinnamates. Hydroxycinnamates include biologically active caffeic acid derivatives, such as 5-O-caffeoylquinic acid (5-CQA) and 3,5-di-O-caffeoylquinic acid (3,5-DCQA), which are potent radical scavengers, lipid peroxidation inhibitors (Ohnishi et al., 1994; Olmos et al., 2008), and neuroprotective agents (Kwon et al., 2010). 3,5-DCQA exerts hepatoprotective effects (Hao et al., 2012) and is active against HIV-1 replication and HIV integrase (Robinson et al., 1996). Numerous pharmacological activities of chlorogenic acid (5-CQA), one of the most ubiquitous metabolites of Asteraceae, have been recently reviewed by Naveed et al. (2018).
Both plants have a long history of medicinal use except as leafy vegetables. Cichorium intybus L. roots and their preparations are used in Europe as traditional herbal medicinal products for the relief of symptoms of mild digestive disorders and temporary appetite loss (European Medicines Agency, 2013). Lactuca virosa L. and Lactuca serriola L. (a wild garden lettuce predecessor) are milky sap (latex) sources. In its condensed form, the latex (known as “lactucarium” or “lettuce opium”) was used as a sedative, analgesic, and antitussive remedy by the middle of the twentieth century (Stojakowska et al., 2000).
Rhizobium rhizogenes-transformed L. virosa and C. intybus roots were obtained using Rhizobium rhizogenes strain LBA 9402.
Lactuca virosa hairy roots produce three structural SLs, lactucin-like guaianolides (8-deoxylactucin, jacquinelin, crepidiaside B, and picriside A), zaluzanin-like guaianolides (macrocliniside A, ixerin F, and scorzoside), and germacranolide (lactuside A; Kisiel et al., 1995). Quantitative analysis of lactucin-like SLs in hairy root biomass demonstrated that 8-deoxylactucin glucoside (crepidiaside A, up to 0.72% DW), jacquinelin glucoside (crepidiaside B, 0.22% DW), and lactucopicrin (0.15% DW) are the major sesquiterpenes in the studied plant material (Stojakowska et al., 2012). Their contents are much higher than those in L. virosa plants (Tamaki et al., 1995). Moreover, 5-CQA (1.1% DW) and 3,5-DCQA (2.6% DW) are the major phenolic constituents of the examined hairy roots and responsible for their potent free-radical scavenging activity. The antiradical activity of the root extract is higher than that of the leaves of the intact plants. The contents of the two caffeic acid derivatives in hairy roots are much higher than those in undifferentiated L. virosa in vitro cultures and the parent plant (Stojakowska et al., 2012). Undifferentiated L. virosa tissue cultures did not produce lactucin-like guaianolides. Their major constituents are neolignans (formerly not found in L. virosa) and caffeic acid derivatives (Stojakowska & Kisiel, 2000; Stojakowska et al., 2012). It is worth noting that Kisiel et al. (1995) first described SL isolation from hairy roots. This study has also been cited by a US patent aimed at treating hepatitis C virus infection with SLs.
Cichorium intybus hairy roots of (Figure 20A), in contrast to undifferentiated C. intybus cultures (Malarz et al., 2005), produce SLs characteristic of the parent plant, including 8-deoxylactucin, lactucopicrin, crepidiaside B, sonchuside A, and ixerisoside D (Malarz et al., 2002). To some extent, exogenous MJ stimulates SL production (Malarz et al., 2007). Further studies on the metabolism of the culture isolated a new natural compound, neolignan [(7S,8R)-3′-demethyl-dehydrodiconiferyl alcohol-3′-O-β-glucopyranoside] (Malarz, Stojakowska, Szneler, & Kisiel, 2013) and identified 3,5-DCQA and 8-deoxylactucin glucoside (5.5% and 1.4%, respectively, calculated on a DW basis) (Figure 20B) as major constituents in long-term C. intybus hairy root cultures (Malarz, Stojakowska, & Kisiel, 2013). Ferioli and D’Antuono (2012) calculated that SL content in two chicory cultivars, Catalogna and Head Radicchio, is maximum 0.22% over dry mass. The radical scavenging activities of C. intybus hairy root extracts are far superior to those of both C. intybus root and aerial part extracts due to caffeic acid derivative (5-CQA and 3,5-DCQA) accumulation in the transformed chicory roots (Malarz, Stojakowska, & Kisiel, 2013).
Total reducing capacity of L. aculeata Boiss. and Kotschy callus tissue is 26.4 mg GAeq/g DW, which is similar to that of C. intybus calli (24.5 mg GAeq/g DW). Chromatographic analysis of a hydroalcoholic L. aculeata undifferentiated tissue extract have demonstrated that mono- and di-caffeoylquinunic acids substantially contribute to the antioxidative activity of cultured cells. The major hydroxycinnamate constituent of the examined plant material (1,5-DCQA) reached 1% DW. Several lignans, formerly known as Lactuca spp. constituents, have been isolated from callus tissue (Stojakowska & Malarz, 2017). Compounds such as (+)-5-methoxybalanophonin, syringaresinol, (+)-buddlenol A, (+)-balanophonin-9-O-β-glucopyranoside (Figure 21), (−)-dihydrodehydrodiconiferyl alcohol-4-O-β-glucopyranoside, and (−)-dihydrodehydrodiconiferyl alcohol-9-O-β-glucopyranoside are active in several in vitro assays as anti-inflammatory and mild cytotoxic agents (Jiao et al., 2011; Woo et al., 2016; Zálešák et al., 2019; Zhu et al., 2015).
Polyphenolic Compounds in Agastache rugosa and Moluccella laevis Shoot Cultures
Phenolic acids and flavonoids, two major phenolic compound classes, have been detected in Agastache rugosa (Fischer & C. A. Meyer) and Moluccella laevis L. in vitro shoot cultures. Light treatment [cool fluorescent lamps, white LED, and photosynthetic active radiation (PAR LED)] and culture conditions influenced the shoot chemical profile. Seven polyphenolic compounds have been identified in Agastache rugosa in vitro shoots using UPLC–DAD–qTOF–MS. RA is the predominant compound, accompanied by only few other phenolic constituents in significantly lower amounts. Plant growth regulator supplementation strongly decreased the RA content in microshoots cultured for 70–105 days than hormone-free media. The RA content increased after culturing for 140 days in hormone-enriched media. Interestingly, culture duration is the most influential factor for RA accumulation (Zielińska et al., 2019). A similar experimental approach was used for M. laevis (bells of Ireland) cell and organ cultures in vitro. Phytochemical analysis revealed the presence of caffeic acid, ferulic acid, and two conjugated caffeic acid derivatives (chlorogenic acid and RA) in shoots and two cyanidin glycosides (cyanidin 3-O-galactoside and cyanidin 3-O-malonylglucoside) in calli. The chlorogenic acid content is the maximum in shoots cultured on MS medium supplemented with 3 µM BA and 0.5 µM IAA under white and PAR LED light conditions (15.5–18.32 mg/100 g DW). It was the first report on the induction of M. laevis shoot and callus cultures using classical biotechnological methods and their specialized metabolite contents (Zielińska et al., 2020).
Volatile Organic Compounds in Agastache rugosa Shoot Cultures
Sixty-five volatile compounds were identified in Agastache rugosa shoot cultures using GC-MS. Phenylallyl volatiles, such as estragole, are almost completely absent in in vitro plant materials. Only the shoots cultured with thidiazuron and picloram emitted 0.7% trans-anethole, whereas at most 0.1% estragole or trans-anethole were detected. Monoterpenes are the most abundant class of volatiles. Shoots grown on culture media without hormone supplementation emitted a large proportion of pulegone (over 90%), whereas adding exogenous plant growth regulators (PGRs) increased monoterpene proportion (20%–50%; Zielińska et al., 2011).
To reveal factors other than the physical or chemical factors influencing in vitro A. rugosa volatile profile, shoots derived from mothers of different ages were examined under controlled conditions. In vitro shoots derived from the seeds of a 1-year-old mother plant emitted a relatively large proportion of volatile compounds that usually do not exceed 1% of the total headspace (sesquiterpenoids, aliphatic alcohols, esters, and ketones). In contrast, shoots obtained from 2- and 3-year-old mother plants abundantly produced the major phenylpropanoid estragole (over 90%). The sum of the five major monoterpene contents (α-pinene, limonene, menthone, isomenthone, and pulegone) in these shoots did not exceed 31%. This was the first record of maternal parentage influence on the in vitro chemical profile of A. rugosa offspring plants (Zielińska et al., 2017).
Isoquinoline Alkaloids in Chelidonium majus In Vitro Cultures
Phytochemical analysis of Chelidonium majus shoots grown on eight different culture media and under two light regimes showed the presence of three isoquinoline alkaloid classes: benzophenanthridine, protoberberine, and protopine. The individual component contents mostly differ between in vitro shoot, root, and callus tissues (Figure 22). The protoberberine derivative, coptisine, is the most abundant compound in shoot cultures, followed by sanguinarine and chelerythrine in roots, while the callus is rich in protopine and sanguinarine. Interestingly, berberine has not been detected in callus cultures and minuscule amounts of coptisine have been detected. This is the first report of isoquinoline alkaloid production in in vitro Ch. majus cell and organ cultures (Zielinska et al., 2018).
. Conclusions
Several plant biotechnological techniques described in this review have opened new perspectives in developing biotechnological protocols for bioactive compound production. Various in vitro culture systems have been developed for medicinal plants belonging to more than dozen botanical families, which produce morphological differentiation-independent family-specific bioactive secondary metabolites (Table 1). Using biotechnological methods such as elicitation, genetic transformation, in situ extraction or permeabilization, and culture media composition optimization allowed us to obtain active compounds in amounts equal to or higher than those in intact plants. Almost all plant species studied were susceptible to genetic transformation using different Rhizobium rhizogenes (Agrobacterium rhizogenes) strains as vectors for desired genes, which developed teratomas, including hairy roots, characterized by the high biosynthetic potential for pharmaceutical compound production. In vitro techniques are expected to help solve some problems associated with medicinal plant species productivity. In future, attention should be paid to large-scale plant tissue and organ cultivation in bioreactors to generate biomass for useful bioactive compound production. Practical applications should also be identified. The most significant advantage of the presented biotechnological approaches is the simultaneous natural environment biodiversity protection, including rare, endemic, or endangered plant species protection.