. Introduction
Diverse processes of haploid and doubled haploid (DH) plant production, including microspore embryogenesis, gynogenesis, and distant crossing followed by genome elimination, are based on the unique ability of plants to form embryos without fertilization, which can be considered a manifestation of plant cell totipotency. All of these are possible only because of various in vitro culture systems that enable the growth and development of tissues or single cells outside of the parental organism. The possibility of redirecting cell development from its original pathway to embryogenesis brings benefits to many research areas, but the most important is the possibility of its implementation in breeding programs.
Breeding of new crop cultivars is a long and costly process, especially in winter-type plants, which require a relatively long time to generate each breeding generation; therefore, it is important to introduce innovative biotechnology methods to accelerate new crop cultivation. In traditional plant breeding, the production of a new cultivar takes an average of 12–15 years and involves many phases of crossing, selection, and testing of the preferred recombinants. The most important advantage of the DH technique is the time reduction in homozygous line production to one generation compared to five–six generations of inbreeding by self-crossing in conventional breeding. In generated DHs, all loci are homozygous, and therefore, all genes are expressed without the phenomenon of dominance. As all traits are genetically fixed, DH lines can be studied in field experiments over many years, and the selection of desired functional traits is much more efficient. As a consequence of genetic homogeneity, DH lines are characterized by morphological uniformity, synchronized time of anthesis and seed setting. Selection allowed to identify both high yielding and high-quality seed producing. Newly obtained genotypes can quickly meet market expectations, ensuring greater profits from the cultivation of modern cultivars.
Thus, understanding the mechanisms controlling the developmental switches involved in DH formation is of great importance and a focus of many scientists and breeders worldwide, including Poland.
The process of “microspore embryogenesis” (ME; synonymous with “androgenesis”) consists of stress-triggered redirection of the development of microspores toward embryogenesis. It was first observed by Guha and Maheshwari in Datura innoxia in 1964 and was the shortest, most uniform, and theoretically the most effective way to achieve total homozygosity (DH plant production). Moreover, microspore suspensions with a density of up to approximately 100,000 per mL of culture medium can serve as a highly advantageous model for various experiments. The system allows for the continuous monitoring of changes in cell structure, as well as for the instant evaluation of modifications introduced in its artificial environment. Microspore suspensions or haploid embryos can also be used as advantageous targets for mutations or transformations, allowing for the production of DH plants homozygous for the mutated/transformed gene. Another in vitro technique, anther culture, is less beneficial as a tool for precise analyses, but because of its simplicity, it has been widely implemented in breeding practice for DH line production.
One more process, “distant crossing” has been used as an alternative method for production of haploid/DH plants. It was introduced by Kasha and Kao in the early 1970s and is also known as the bulbosum method (Kasha & Kao, 1970). This process is based on uniparental chromosome elimination during the early stages of hybrid embryo development, leading to the formation of a haploid embryo. It was first reported as a result of the interspecific hybridization of cultivated barley (Hordeum vulgare L.) with H. bulbosum (L.); however, other distant crosses, such as wheat × maize, wheat × barley, or oat × maize, have been used to produce haploids in cereals. Maternal haploid induction is the most commonly used technique for maize DH production, mainly because of the discovery of inducer lines, which allow identification of haploid plants by their expression of the anthocyanin color marker R1-nj (Navajo phenotype; Chaikam et al., 2015).
In addition, ovules, ovaries, or flower buds can switch from their normal gametophytic pathway toward sporophytic development, which is described as gynogenesis. Compared to ME, the gynogenic strategy to produce haploids seems to be less effective, but still advantageous in species with male sterility, a high frequency of albino plants, or that are recalcitrant to microspore reprogramming. Direct haploid parthenogenesis, in which female gametophytes serve as the origin of haploid cells, is beneficial, but plant regeneration can be obtained indirectly by calli proliferating from induced haploid embryos. The first in vitro haploid plants of gynogenic origin were obtained from barley (San Noeum, 1976). Since then, advances have been made in other species.
Many years of research have brought significant progress in the understanding of the mechanisms that redirect cell fate toward embryogenic development. However, the precise mechanism underlying this phenomenon has not yet been definitively identified. For example, despite the huge theoretical potential of ME, in practice usually only a small fraction of in vitro cultured microspores switch to the embryogenic pathway. Common bottlenecks are the low regeneration potential of microspore-derived embryo-like structures (ELSs) and, in the case of monocotyledons including the most important cereals, the high frequency of albino regenerants, which are unable to survive ex vitro (Wędzony et al., 2009). The problem is the number of internal and environmental factors and the complexity of their interactions involved in determining the course and effectiveness of the process. This technology can be incorporated into breeding practices in only a limited number of plant species. There is a need for new data and insights to break down these barriers and enable the development of more efficient procedures.
This review summarizes the achievements of Polish research groups in examining the mechanisms of non-zygotic haploid/DH embryo development and demonstrates the practical applications of these systems in basic plant studies and breeding. The work described here has made an important contribution to our understanding of the complex mechanisms of non-zygotic embryo formation leading to DH plant formation. The outcome has been the development of several efficient methods for obtaining DH lines, mainly in cereals and Brassicaceae species, which are very useful in modern plant breeding.
. Triticale (×Triticosecale Wittm.) as the Plant Model for Studies on Microspore Embryogenesis
Triticale is an artificial species that originated from a cross between wheat and rye, and undertaken with the hope that it would combine the high grain quality of Triticum with the vigor and high adaptability to adverse environmental conditions of Secale. These expectations were not completely fulfilled; moreover, the complex genetic organization resulted in genetic instability and genomic changes. Effective DH technology, which provides totally homozygous models for basic studies and can be used as an advanced tool in breeding, would seem to be the most promising way to further improve this species.
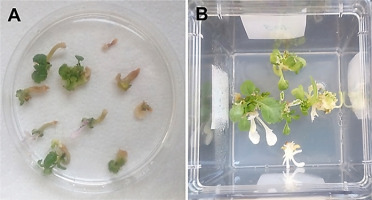
Various Polish triticale cultivars have been the foci of ME studies conducted in the Department of Cell Biology at the Franciszek Górski Institute of Plant Physiology Polish Academy of Sciences (IPP PAS). As a result of these studies, both anther culture and isolated microspore culture protocols (Figure 1) have been optimized and published by Wędzony (2003) and Żur (2007). The establishment and evaluation of 90 DH lines of winter triticale from the mapping population ‘Saka 3006’ × ‘Modus’ started a new chapter in the research, providing a precisely defined model applicable to many areas of research. The 3-year-long set of anther culture experiments in various vegetation seasons allowed the selection of DH lines that stably and significantly differed in their embryogenic potential and the identification of quantitative trait loci (QTL) associated with responsiveness to ME-inducing treatments (Krzewska et al., 2012, 2015). In addition, the selected DH lines also showed high variation with respect to ME effectiveness in isolated microspore cultures (Żur et al., 2019).
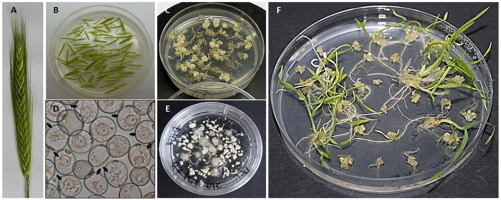
Figure 1
Microspore embryogenesis (ME) in anther culture and isolated microspore culture of triticale (×Triticosecale Wittm.). (A) Triticale spike at the stage optimal for ME induction. (B) Isolated triticale anthers transferred to the induction medium. (C) Embryo-like structures (ELSs) after 6 weeks of in vitro culture. (D) Star-like structures (arrows) in isolated triticale microspore cultures after transfer to the induction medium. (E) ELSs produced after 6 weeks in vitro co-culture with immature ovaries. (F) Plantlets regenerated after transfer to the regeneration medium.
In the last decade, investigations conducted in the Department of Cell Biology of IPP PAS have aimed to understand the role of reactive oxygen species (ROS) and cellular redox potential in ME induction. ROS are products of the partial reduction of molecular oxygen produced in all living cells as an unavoidable by-product of aerobic metabolism (Mittler, 2002). Their abundance depends on the dynamic equilibrium between the intensity of ROS generation and efficiency of the ROS-scavenging system. Intensive accumulation of ROS can be lethal but it can also be a trigger that initiates stress defense strategies (Miller et al., 2010). Our first study on two spring triticale cultivars revealed that various stress factors used for ME initiation affect energy metabolism (respiration rate and heat emission) and the activity of antioxidant enzymes (superoxide dismutase, catalase, and non-specific peroxidase). The response was genotype-specific and associated with the effectiveness of ME induction (Żur et al., 2008, 2009). To verify these results, the generation of ROS [superoxide anion (O2⋅−) and hydrogen peroxide (H2O2)] and the activity of both enzymatic and non-enzymatic antioxidants were estimated in eight DH lines of winter triticale that significantly differed in their embryogenic potential (Żur, Dubas, Krzewska, Janowiak, et al., 2014). The crucial requirement for high efficiency ME was found to be the ability to increase or at least sustain the activity of antioxidant enzymes during low-temperature tiller treatment (3 weeks at 4 °C) used for ME induction (Żur, Dubas, et al., 2021). Based on the positive correlation between ME effectiveness and ROS generation (r = 0.85), it was suggested for the first time that these molecules play an important signaling role and that their generation is necessary for successful ME initiation (Żur, Dubas, Krzewska, Janowiak, et al., 2014; Żur, Dubas, et al., 2021). It has also been shown that non-enzymatic antioxidants cannot efficiently substitute for the enzymatic defense system against ROS (Żur, Dubas, Krzewska, Janowiak, et al., 2014). However, one of the most important low-molecular-weight antioxidants, glutathione, the key regulator of cellular redox homeostasis, seemed to be involved in the subsequent stages of ELS development (Żur et al., 2019). The effects of tiller treatment with reduced glutathione (GSH) varied depending on the stress intensity and activity of endogenous antioxidative systems. This treatment sustained microspore viability because the strongly reduced intracellular environment lowered ROS accumulation and decreased the intensity of oxidative stress. However, ROS elimination suppressed the transduction of the signal necessary for microspore reprogramming (Żur, Dubas, et al., 2021; Żur et al., 2019). In contrast, a more oxidized environment (lower redox potential) promoted the next stages of ELS formation. These findings shed new light on the role of ROS as common signaling molecules involved directly in microspore reprogramming and ME induction (Figure 2).
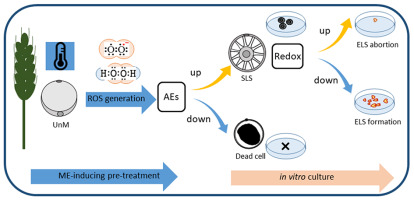
Figure 2
Determinants of successful induction of microspore embryogenesis (ME) in isolated microspore cultures of triticale (×Triticosecale Wittm.). Low temperature treatment (3 weeks at 4 °C) – a standard trigger of ME – applied to tillers containing uninucleate, totipotent microspores (UnM) is associated with generation of reactive oxygen species (ROS), necessary for microspore reprogramming. High activity of antioxidative enzymes (AEs) sustains microspore viability and allows for effective ME induction (visible in high number of star-like structures, SLS). Decreased activity of AEs frequently results in cell death. However, even high viability of microspores does not ensure final success as the next stages of embryogenic development require a more oxidized environment (lower redox potential).
Our other studies have focused on the hormonal regulation of ME. The role of phytohormones, mainly auxins (Auxs) and cytokinins (CKs), in ME has been widely studied and described by many authors (reviewed by Żur, Dubas, Krzewska, & Janowiak, 2015), but our focus was a unique analysis of endogenous homeostasis and crosstalk between Aux, CKs, and abscisic acid (ABA) associated with the acquisition of microspore totipotency. It was revealed that low temperature tiller treatment (3 weeks at 4 °C), used as a standard for ME induction in triticale, was accompanied by significant changes in the levels of all analyzed phytohormones. Lower values of Aux/CKs, Aux/ABA, and CKs/ABA ratios, as well as a proper balance between endogenous Auxs and Auxs supplied by culture media, were crucial for highly efficient ELS formation and green plant regeneration (Żur, Dubas, Krzewska, Waligórski, et al., 2015).
Several DH lines of triticale have also been used in studies on genetic and epigenetic control of microspore reprogramming. It has been shown that some wheat orthologous genes identified earlier as being involved in microspore-derived ELS development (Sánchez-Díaz et al., 2013) could also be found in triticale and that their expression pattern was related to efficiency of ME induction (Żur, Dubas, Krzewska, Sánchez-Díaz, et al., 2014). Among them were genes associated with signaling, control of cell wall modification, cell pattern formation, intra-embryo communication, and differentiation, as well as genes involved in oxidative stress defense, including glutathione transferases (GSTF2, GSTA2), chitinase (CHI3), and small cysteine-rich proteins similar to plant defensins or thionins (Tad1). Changes in their expression associated with ME-inducing stress treatment support our hypothesis regarding the role of ROS in the control of ME at the molecular level. Other genes that were identified regulate the biosynthesis of auxin (TAA1b) and auxin-responsive elements of the network that direct somatic plant cells toward embryogenic development (AGL14 and SERK).
The expression of genes related to ME (TaTPD1-like, GSTF2, GSTA2, CHI3, Tad1, TaNF-YA7, SERK2, and TaME1) was dysregulated by the application of two inhibitors of DNA methylation (5-azacytidine and 2′-deoxy-5-azacytidine), which confirmed the role of epigenetic processes in triticale microspore reprogramming (Nowicka et al., 2019). Although the effect of DNA hypomethylation was genotype-dependent, the applicability of epigenetic inhibitors to improve the effectiveness of triticale DHs production has been postulated.
Our studies also revealed that ME induced by low-temperature tiller treatment (3 weeks at 4 °C) is associated with changes in the triticale anther proteome (Krzewska et al., 2017). Most changes revealed in protein profiles were proteins involved in cell metabolism (47%), stress response (28%), protein synthesis and storage (9% and 6%, respectively), energy metabolism (6%), and active cell division (3%). In embryogenic microspores, proteins that were significantly more abundant included proteins that act to ensure proper metabolism and energy production, such as beta-amylase, fructokinase-2, oxygen-evolving enhancer protein 1, ribulose-1,5-bisphosphate carboxylase/oxygenase, and ATP synthase CF1 beta subunit. Evidently, important roles are played by proteins regulating biosynthesis of phytohormones (S-adenosyl-l-methionine synthase) and determining stress adaptation ability (elicitor responsive protein 3, heat shock cognate 70 kDa protein 4, putative aconitate hydratase). Two protein species (enolase and 12S storage protein) have been proposed as potential markers of ME. Further studies (Krzewska et al., 2021) have shown that tiller treatment with 5.0 µM 5-azacytidine that stimulated ELS development also alters the protein profile of triticale anthers. The most important modifications suggested a switch from anabolic to catabolic metabolism (downregulation of chlorophyll a–b binding protein, coproporphyrinogen-III oxidase, RuBisCO large subunit-binding protein subunit beta, and oxygen-evolving enhancer protein 1 concomitant with upregulation of glyceraldehyde-3-phosphate dehydrogenase, enolase, and phosphoglucomutase), as well as more effective protection of proper protein folding (heat shock 70 kDa) and degradation of dysfunctional or damaged proteins (26S proteasome non-ATPase regulatory subunit 7).
The data collected not only increase our knowledge of the mechanisms regulating microspore reprogramming and ME induction in triticale, but also provide the basis for practical improvement of in vitro culture protocols for other, more recalcitrant crops such as rye (Zieliński et al., 2020).
. Molecular Mechanisms Leading to Albinism in Cereal Androgenesis
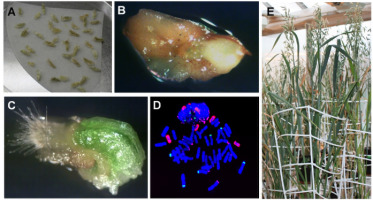
Albinism, which occurs in anther and microspore cultures of many monocot species, is a fundamental problem that limits the utilization of androgenesis in breeding programs for cereals and grasses (Żur, Gajecka, et al., 2021). The lack of chlorophyll (albinism) present in a significant proportion of regenerated plants can significantly reduce the final DH production efficiency (Figure 3). Albino plants contain non-functional chloroplasts, which means that they are only able to grow in vitro.
Figure 3
Development of embryos and regeneration of plants in isolated microspore culture of barley (Hordeum vulgare L.). (A) Mid-to-late (ML) microspores after isolation in mannitol solution. (B) Induced microspore-derived embryos on twenty-first day of in vitro culture. (C) Differentiating embryos on thirty-fifth day of in vitro culture. (D,E) Regenerating green (D) and albino (E) plantlets. (F) Representative regeneration dishes of five barley genotypes presenting: 90%–70% (‘Jersey,’ ‘Bruce’), ca. 50% (‘Skald,’ ‘KWS Aliciana’), and 5% of green regenerants (‘Mercada’).

Microspores contain proplastids that can differentiate into all types of plastids (Jarvis & López-Juez, 2013). During in vivo development of microspores into pollen grains, proplastids differentiate into amyloplasts that accumulate starch (Clement & Pacini, 2001). These proplastids form fully functional chloroplasts during androgenesis. Nevertheless, some microspore proplastids fail to differentiate and remain arrested early in their differentiation, leading to the regeneration of albino plants (Caredda et al., 2000). Albinism is a highly genotype-dependent phenomenon that occurs in anther and isolated microspore cultures of all the main cereals, including wheat (Lantos et al., 2006), rice (He et al., 2006), barley (Makowska et al., 2015), and triticale (Lantos et al., 2014). In some genotypes, the frequency of albino plants can reach almost 100%, which excludes the utilization of these genotypes in DH-based breeding programs. Several attempts have been made to explore various hypotheses to identify the causes of albino development. One of these concerns a change in plastid genome structure, mostly deletions, identified in albino regenerants of barley (Day & Ellis, 1985), triticale (Mozgova et al., 2012), wheat (Day & Ellis, 1984) and rice (Yamagishi, 2002). The size and location of deletions differed among albino regenerants, but most deletions appeared in the long single-copy region (LSC) of the plastid genome, which is the most unstable part of the plastome (Dunford & Walden, 1991). The LSC region contains genes that are involved in photosynthesis, such as genes encoding the subunits of photosystems I and II. Moreover, the study of Ankele et al. (2005) demonstrated that only some of the albino plants regenerated from wheat anther culture carried changes in the plastome structure, but all of them exhibited altered transcriptome profiles compared to the green regenerants. The differential display analysis of green and albino regenerants showed a decreased expression level of genes related to photosynthesis, for example, RbcS encoding a small subunit of RubisCo, and higher expression of genes involved in plastid biogenesis in albino plants (Ankele et al., 2005). Additionally, in barley (Muñoz-Amatriaín et al., 2008) and triticale (Krzewska et al., 2015), QTLs associated with the frequency of green plants regenerated from androgenic cultures have been identified. However, the molecular mechanisms leading to the alteration of chloroplast development in albino regenerants remain unclear, and none of the proposed hypotheses explain the genotype-dependent regeneration of albino plants.
At the Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Sciences, University of Silesia in Katowice, we conducted studies aimed at identifying the molecular processes leading to the genotype-dependent formation of albino regenerants in isolated microspore culture of barley (Hordeum vulgare L.). Using spring barley cultivars ‘Jersey’ and ‘Mercada,’ which both exhibited a high regeneration potential but differed in their ability to regenerate green plants during androgenesis, we showed that the state of plastid differentiation in microspores at the time of material collection determines their fate during in vitro culture (Gajecka et al., 2020). The cultivar ‘Mercada,’ which produces more than 90% albino regenerants, exhibited a faster differentiation of proplastids into amyloplasts during pollen development in vivo. In the microspores of this cultivar, at the medium-late mononuclear (ML) stage, which is routinely used for culture initiation (Dunwell, 2010), nearly half of the plastids are differentiated into amyloplasts. The remaining half consisted of proplastids and only a few initial proplastids. In contrast, microspores of ‘Jersey,’ that produced mostly green plants in androgenic culture, at the same ML stage contained mostly undifferentiated initial proplastids, which showed a low electron density when examined using transmission electron microscope (TEM). Amyloplasts in ‘Jersey’ were not observed till the binucleate immature pollen grain stage. The hypothesis of early amyloplast formation in albino-producing genotypes was verified by the analysis of several barley cultivars that had highly divergent frequencies of albino plant regeneration, varying between 10% and 98%. Cultivars that mostly produced albino regenerants in in vitro culture harbored a high number of amyloplasts in ML microspores, whereas in cultivars with a high ability to regenerate green plants, only initial proplastids and a few differentiating proplastids were identified. The number of albino plants regenerated from microspore culture was strongly correlated with the amyloplast number in the ML microspores (r = 0.94) and negatively correlated with the number of differentiating proplastids (r = −0.88) (Gajecka et al., 2020).
Together with the presence of amyloplasts, we observed degradation of the plastid genomes, as demonstrated by a decrease in the average number of plastomes between the early (E) and mid-to-late (ML) stages of microspores of ‘Mercada,’ as well as a divergent number of plastid genes located throughout the plastid genome.
The occurrence of amyloplasts was preceded by an increase in the expression levels of genes encoding enzymes of reserve starch biosynthesis in pollen grains, including the Sbe1 (starch branching enzyme1), Dpe2 (4-alpha-glucanotransferase2), and granule-bound starch synthase I (GBSSI) genes. In ‘Mercada’ that produced mostly albino regenerants, expression of these genes increased to high levels as early as the early-to-mid (EM) microspores, whereas in ‘Jersey’ the increase was observed only in the ML microspores. When the expression of these genes was analyzed in 10 barley cultivars differing in their ability to produce green regenerants, a very strong positive correlation was observed between the expression levels of the Dpe2, GBSSI, and Sbe1 genes in the EM microspores and the rate of regenerated albino plants in the in vitro culture. This analysis allowed us to distinguish GBSSI gene expression in EM microspores as a valuable marker of amyloplast differentiation, which indicated the genotypes that would produce mostly albino regenerants in isolated microspore culture. It can be concluded that the faster conversion of proplastids into amyloplasts, which results in a high proportion of amyloplasts in ML microspores, is associated with the formation of albino plants during androgenesis.
Based on the correlation between the early formation of amyloplasts during microspore development and the proportion of albino plants in androgenic culture, we assumed that the initiation of in vitro cultures from an earlier stage of microspore development might positively influence the ratio of green to albino regenerants. To test this hypothesis, the microspore cultures of some cultivars expressing very high frequency of albino regenerants (ca. 90%) were initiated from the microspores at the EM stage of development, which harbored the initial proplastids only. The frequency of green plant regeneration increased significantly, on average, from 12.6% to 46.6%, which allowed approximately 60 plants per 100,000 microspores (i.e., in a Petri dish 3 cm in diameter) to be obtained. This indicates that the initiation of cultures from microspores containing proplastids prior to amyloplast differentiation can significantly improve the regeneration of green plants and overcome the problem of albinism during barley androgenesis (Gajecka et al., 2020).
Comparison of plastid differentiation between cultivars ‘Jersey’ and ‘Mercada’ during ME and plant regeneration enabled us to gain insights into the molecular mechanisms leading to the formation of albino plants (Gajecka et al., 2021). During induction of ME, the expression of Dpe2, GBSSI, and Sbe1 genes involved in reserve starch biosynthesis decreased in both cultivars, which indicates that the application of stress treatment before in vitro culture inhibited reserve starch synthesis during induction of ME. In contrast, the expression of GBSSIb and SSIIb encoding enzymes involved in assimilatory starch synthesis increased gradually after pre-treatment in both cultivars. However, in ‘Mercada’ the increase in the number of amyloplasts was observed in developing embryos before activation of assimilatory starch synthesis genes, which indicated that the amyloplasts harbored by the microspores at the stage of culture initiation were capable of division. During embryo differentiation, between the twenty-first and thirty-fifth days of in vitro culture, a significant decline in plastome copy number was observed in both cultivars. The number of plastome genomes increased in ‘Jersey’ during the further phases of embryo development but remained at a low level in ‘Mercada’ embryos. Additionally, in ‘Mercada,’ apart from the decline of plastome copies, the numbers of individual plastid gene copies also varied and differed from the values expected based on the gene location within the plastome.
The plastid genome of ‘Mercada’ shows high instability during embryo development. We assumed that a low number of correct plastome copies would influence the differentiation of proplastids into chloroplasts. A high number of correct plastid genomes is considered a checkpoint for chloroplast differentiation (Maréchal & Brisson, 2010). Possession of a high number of plastomes, together with the effective transcription machinery, provides enough plastid rRNAs transcripts for chloroplast differentiation. Transcription in plastids is performed by two RNA polymerases: NEP nuclear-encoded polymerase (NEP) and plastid-encoded polymerase (PEP) (Yagi & Shiina, 2014). NEP and PEP recognize specific promoters and transcribe a specific set of genes, although their specificities partially overlap. In the early stage of plastid biogenesis, NEP, a single-subunit phage-type RNA polymerase, is the major RNA polymerase for the transcription of plastid-localized genes involved in plastid biogenesis. As proplastid-to-chloroplast differentiation progresses, the major role in gene transcription in plastids is acquired by the bacterial-type PEP polymerase. PEP consists of five subunits: two α and β, β′, and β″ encoded by the plastid rpoA, rpoB, rpoC1, and rpoC2 genes located in separate operons. The proper action of PEP depends on sigma factors, which are encoded by the Sig1–Sig6genes located in the nuclear genome and transported into the plastid. Individual sigma factors recognize specific promoters and enable transcription initiation by the PEP holoenzyme (Pfannschmidt et al., 2015). The transition of transcription polymerases is regulated by the SIG2 factor, which after import to the plastid, forms a complex with the PEP and initiates the transcription of tRNAGlu. High levels of tRNAGlu transcripts inhibit NEP activity by binding to it (Hanaoka et al., 2005). It should be stressed that only PEP-dependent transcription of plastome rRNA supplies sufficient rRNA molecules for the assembly of plastid ribosomes and translation of chloroplast proteins (Börner et al., 2015). At the Institute of Biology, Biotechnology and Environmental Protection, University of Silesia in Katowice, we analyzed the expression of nuclear and plastid genes involved in chloroplast biogenesis (including Sig2 and tRNAGlu) during embryo differentiation and plant regeneration in ‘Jersey’ and ‘Mercada’ cultures. We revealed that during the conversion of ‘Jersey,’ the PEP-dependent transcription was activated between the forty-third and forty-sixth days of culture, based on the observed 37-fold increase of Sig2 and 5-fold increase of tRNAGlu transcripts within this period. In contrast, in ‘Mercada’ we observed a relatively low expression level of tRNAGlu throughout the whole period of plant regeneration, which indicated that in the absence of PEP activity, the NEP was still the dominant RNA polymerase in ‘Mercada’ plastids. As a consequence of the failed NEP-to-PEP transition, no significant increase in plastid rRNAs was observed in differentiating embryos and regenerated plantlets of this cultivar. In contrast, in ‘Jersey,’ the relative expression of 16S and 23S genes encoding plastid rRNAs increased 20–30-fold between the forty-third and forty-sixth days of culture and reached 300–500 times higher levels in the regenerated plantlets on the fifty-fifth day of culture. Consequently, in ‘Mercada’ there was no activation in the regenerating embryos and albino regenerants of the transcriptional factors GLKs, which are the positive regulators of photosynthesis-associated nuclear genes (Gajecka et al., 2021).
. The Development of DHs Technology for Cereal Species Through Selection of New Varieties With High Embryogenic Potential and Its Implementation to Breeding Practices
In the nineties, several DH methods, including the bulbosum method, anthers culture for barley and wheat, and isolated microspores culture for barley and triticale were developed for the most important cereal species by the Department of Biotechnology and Cytogenetics of Plant Breeding and Acclimatization Institute – National Research Institute (PBAI-NRI) in Radzików (Czembor et al., 2003; Konieczny et al., 2005; Makowska, Kałużniak, et al., 2017; Makowska & Oleszczuk, 2014; Makowska et al., 2015, Makowska, Oleszczuk, & Zimny, 2017; Oleszczuk et al., 2004, 2006). Every year, we produce several thousand DHs of various cereal species, which are sent to breeding companies where they are successfully used in breeding programs.
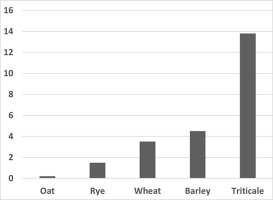
As part of our research on DH production, we compared the androgenic capacities of various economically important cereal species, kindly provided by Polish plant breeding companies. Enormous variation in androgenesis capacity exists between different plant species. Based on our experience, a hierarchy of cereals has emerged in terms of their morphogenic capacity in anther cultures (Figure 4). In this respect, Triticale was always considered the most responsive of the studied species. Wheat was more recalcitrant than barley, whereas rye and oat were the most difficult species in which to initiate androgenesis.
Figure 4
Regeneration effectiveness (number of green regenerants per 100 anthers cultured) of various cereals. Anthers were collected from 23 to 30 lines from each species, and from at least 20 spikes per line.
Highly efficient regeneration was achieved. For example, during three seasons of production, 51 crossing combinations of triticale over 19,000 DH lines were regenerated and transferred to breeding companies. Large differences were observed in the effectiveness of regeneration between genotypes and spikes. The values varied from 0.4 to 98 green regenerants per spike (on average, 19 green regenerants per spike).
To optimize the performance of the in vitro culture method, we used Taguchi’s method, which is applicable when multiple factors are subject to simultaneous optimization, and the use of a complete plan is uneconomical owing to the size of the experiment. Importantly, the application of appropriate statistical data analysis based on statistical methods and the use of Taguchi’s method with orthogonal arrays can reduce the number of experiments needed for optimization from 27 to nine experimental variants (for three factors at three levels) compared to the example of the complete plan (Orłowska et al., 2020).
Although the regeneration of DH plants of triticale has been achieved for many cultivars, there are still some challenges in enhancing the efficiency of androgenesis. In previous years, we have conducted research on various aspects of homozygous plant production.
Our studies indicate that some regenerants among populations of DH triticale lines are clones formed through secondary embryogenesis in the callus stage or through the formation of twin/polyembryos (Oleszczuk et al., 2014). The scale of this phenomenon reduces the genetic variation generated via androgenesis and restricts the selection of genetically unique lines for breeding purposes. This knowledge should be considered when modifying DH regeneration protocols to reduce the number of clonal replicates.
Another problem with triticale androgenesis is the low frequency of spontaneous diploidization among regenerants. We found that crossing parental lines with DH can increase not only the percentage of spontaneous chromosome doubling but also androgenesis efficiency (Oleszczuk et al., 2021). Meiotic restitution can also increase the number of fertile androgenic regenerants. In the present study, a mapping population of microspore-derived haploids from an F1 wheat-rye hybrid was created (Oleszczuk et al., 2019). Further studies on the localization of the loci responsible for meiotic restitution should be undertaken for the practical application of this process in the doubling of chromosomes in haploids.
Although commercial triticale cultivars are meiotically stable, poor chromosome pairing in breeding hybrids results in the occasional occurrence of univalents (Oleszczuk & Banaszak, 2016). Our results showed that aneuploids are obtained in high proportions during triticale androgenesis (Oleszczuk et al., 2011), further reducing the efficiency of the process. Aneuploidy may also occur in advanced breeding lines, creating stability issues in variety registration.
The published methodologies for homozygous lines provide the opportunity to conduct advanced basic research and breeding with a new tool for plant regeneration from isolated microspores (Oleszczuk et al., 2004), stabilizing genotypes, and increasing plant breeding productivity (Tyrka et al., 2018; Warzecha et al., 2005).
The methodology for producing DHs has been successfully implemented by breeding companies, resulting in the development of new cultivars. This has been verified by registering at least two triticale cultivars (‘Borowik’ and ‘Panteon’ – Plant Breeding Strzelce Ltd. Co., PBAI-NRI Group) of high agronomic importance. ‘Borowik’ is a winter triticalethat can be grown for grain, fodder, green matter, straw for animal production, or for combustion for heating biofuel (ethanol). The second cultivar ‘Panteon’ has the highest protein content among triticale (bread triticale), yielding protein at the level of 110%–120% of the standard. It is resistant to soil acidification, has low soil requirements, and exhibits very good winter hardiness.
Our achievements were appreciated by the Minister of Agriculture and Rural Development through the granting of “The Award of the Minister of Agriculture and Rural Development for achievements in the implementation of progress in agriculture” in 2011.
Barley is considered to be a model species among monocot plants for a wide range of biological experiments. After developing (between 2000 and 2004) and describing the method of isolated microspore culture, the methods were applied to an in-depth study of various aspects of barley androgenesis. We focused on three aspects that we believe are important for barley androgenesis: (i) the genotype of the donor plant as an element that largely determines the effectiveness of the process, (ii) an attempt to increase the abundance of regenerated androgenic plants, and (iii) the reduction of undesirable albinism.
The formation of androgenic structures with low regeneration potential is a common event observed during androgenesis in barley genotypes. Attempts have been made to increase the conversion rate of such structures into plants by the application of gum arabic to the induction medium (Makowska, Kałużniak, et al., 2017). Gum arabic is a mixture of compounds including arabinogalactan proteins, which are involved in restoring and maintaining the embryogenic potential of cells in vitro. The results obtained confirmed the positive effects of gum application on microspore survival during the initial days of in vitro culture and on morphology of androgenic structures, which led to more efficient plant regeneration.
Albinism is common among androgenesis-derived plants, including most cereals, and the percentage of albino plants varies from 1% to 99.7%, depending on the genotype. Genetic studies have identified loci that correlate with the appearance of albino plants produced during androgenesis (Muñoz-Amatriaín et al., 2008). However, there are many reports confirming the effect of specific in vitro culture conditions on the frequency of regenerated albino plants. Our review paper discusses issues related to albinism, including its sources, the extent of occurrence among different genotypes, and ways to reduce the number of albino plants in in vitro cultures (Makowska & Oleszczuk, 2014).
Within the same species, there are often cultivars that are more susceptible to albinism. Studies conducted on a large and genetically diverse group of spring and winter barley genotypes have shown that the characteristics of being a winter crop are positively correlated with regeneration effectiveness (Makowska & Oleszczuk, 2014). Among the winter genotypes, it was possible to obtain a significantly higher number of green plants, as well as a lower level of albinism, compared to the spring genotypes (Makowska et al., 2015). The type of plant development (spring/winter) was not significant for obtaining regenerants capable of spontaneous doubling of the ploidy.
Studies on the influence of elevated Cu ion content in induction media on the decrease in the regeneration percentage of chlorophyll-free plants were performed by Makowska, Oleszczuk, and Zimny (2017). For both tested lines, the number of androgenic structures produced was not significantly different between the control and the media enriched with additional copper ions, but the mean total plant regeneration effectiveness was improved by 34%. This result was due to the higher number of regenerated green and albino plants, whereas the ratio of the green and chlorophyll-free regenerants obtained in both genotypes tested remained at the same level (1:3), regardless of the composition of the medium.
Over many years, we have tested many genotypes, stresses, and in vitro culture conditions on rye, which is considered a very difficult and resistant species in which to induce androgenesis. A major success of our laboratory in the past few years has been the selection of rye lines with an unprecedented high capacity for androgenesis (J. Zimny, personal communication, 2019; Figure 5). These lines have become model genotypes for studies on rye androgenesis, making it possible to test the determinants of androgenesis in a reproducible manner. The optimum stress conditions to induce microspore division and DH regeneration in rye were determined. Cooling of shoots with spikes for a period of 21 days, as well as cooling of shoots with spikes for 14 days in combination with subsequent incubation of anthers in mannitol solution, were considered the most appropriate type of stress. The method for isolated microspore culture in rye was mastered, and factors favorable for this culture were investigated. This led to the discovery of the positive effect of monochromatic light on anther cultures of rye, which until now has usually been carried out in the dark (Zimny & Michalski, 2019; Zimny et al., 2021).
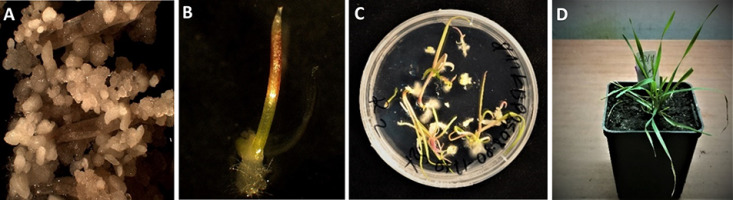
Figure 5
Illustration of rye androgenesis from callus with androgenic ELS to regenerated plant. (A) Embryogenic callus and clearly differentiated embryos and ELS. (B) Germinating androgenic embryo. (C) Green plantlets on regeneration medium. (D) Rooted DH of rye.
The analysis of phenomena that determine the induced variability in in vitro cultures has important research and practical applications (Bednarek et al., 2007; Oleszczuk et al., 2011). Research has focused on the composition and complex background underlying these phenomena, such as morphological, biochemical, and molecular aspects, and the interactions between some of the determinants (Machczyńska, Orłowska, Mańkowski, et al., 2014; Machczyńska, Orłowska, Zimny, & Bednarek, 2014; Machczyńska et al., 2015). It is important to know what factors affect the formation of induced variability in in vitro cultures, and how the level of this variability or the effectiveness of green plant regeneration can be regulated (Orłowska et al., 2016, 2021). Hence, the practical aspect is the possibility of influencing the phenomenon of induced variability in in vitro cultures. Another option with practical implications is the optimization of in vitro cultures, as it creates the possibility of modifying the conditions of running the cultures depending on experimental needs without going more deeply into the research aspect (Orłowska et al., 2020).
Our methods have been implemented by several breeding companies and have become an important part of the breeding process. In addition to studies in applied botany, we are also involved in basic research in which we are trying to understand the mechanisms governing generative reproduction, such as restoring fertility in haploid plants (Oleszczuk et al., 2019).
. DHs of Winter Oilseed Rape (Brassica napus L.) in Plant Breeding and Applied Research
In the 1980s, it was shown that it is possible to obtain many microspore-derived embryos in isolated microspore cultures of oilseed rape, bypassing the proliferation phase of callus tissue in a liquid NLN medium without the addition of hormones (Lichter, 1982). Currently, protocols for producing DHs from in vitro isolated microspore cultures of Brassica napus L. differ between laboratories, but the basic rules of the procedures remain the same. In this method, efficient ME is induced by high-temperature (30–35 °C) stress treatment of microspores immediately after isolation. The main stages of in vitro culture are the isolation of microspores from donor plant anthers, doubling of chromosome number, and stimulation of microspore-derived embryos for plant development.
Achievements in research on this technique carried out at the Tissue Culture Laboratory in Plant Breeding and Acclimatization Institute – PBAI-NRI in Poznań Division (IHAR-PIB), provided oilseed rape breeders with a method of quickly obtaining a large number of completely homozygous genotypes, and offered researchers an excellent resource for many directions of studies on winter oilseed rape (Cegielska-Taras et al., 2002, 2015). This method was previously described in detail by Cegielska-Taras et al. (1999), and Cegielska-Taras (2004), and is still being improved and includes many variants, depending on the susceptibility of individual genotypes to in vitro ME. Particularly important points of our method, not previously found in other protocols, are: (i) use of NLN-13 medium (Lichter, 1982) in the entire isolation process, (ii) treatment of microspores with 0.05% colchicine solution for 20–22 hours, starting immediately after their isolation, (iii) stimulation with a high concentration of kinetin (10−4 M), which allows for the development of shoots from androgenic embryos in the process of organogenesis (Figure 6).
Figure 6
Stages of doubled haploid production of oilseed rape (Brassica napus L.) through isolated microspore culture. (A) Inflorescence of donor plant at the stage optimal for isolation of microspores. (B) Three days of isolated microspore culture – first microspore divisions. (C) Microspore-derived embryos (MDEs) after 10 days of in vitro culture. (D) MDEs after 3 weeks of in vitro culture. (E) Apical shoot development – 5–6 weeks of in vitro culture. (F) Shoot regeneration. (G) Plant rooting. (H) Androgenic plants transferred to soil after vernalization. (I) Flowering plants in a greenhouse. Photographs from the archives of the Department of Oilseed Crops, Plant Breeding and Acclimatization Institute – National Research Institute.

Treatment of freshly isolated microspores with colchicine results in a doubling of the number of chromosomes in the range by 16%–94%, depending on the donor plants (Szała et al., 2020). In addition to doubling the number of chromosomes at the in vitro culture stage, methods have also been developed for doubling chromosome number in young haploid plants in vivo by dipping their roots or axillary shoots in colchicine solution (Szała et al., 2020). This has made it possible to double the chromosome number of each haploid winter oilseed rape plant. The method of obtaining DH lines through isolated microspore culture is currently an integral part of the breeding programs for oilseed rape in Poland. The protocol is characterized by high repeatability and efficiency in the mass production of DHs from different donor plants. This method is easily adaptable to the conditions of many laboratories. In 2005, this protocol was implemented in the Plant Breeding Company Strzelce Ltd., Division Borowo, and later in Division Małyszyn, where thousands of DHs of oilseed rape are obtained annually. Owing to the introduction of the method of doubling the number of chromosomes in microspores immediately after their isolation and the use of the method of stimulating embryos to develop into plants, this method is characterized by the low cost of obtaining each DH line.
The DH line could be a potential new cultivar. As a result of 5 years of breeding work, the DH MA-103 line was selected by the Plant Breeding Company Strzelce Ltd. in 2005 and added to the Polish National List (NLI) in 2008 as the first Polish open-pollinated cultivar ‘Monolit’ (single DH line) of winter oilseed rape (Cichy et al., 2005). This cultivar is characterized by a high and stable yield and a high fat content in the seeds. It has low soil requirements and strong resistance to fungal diseases, particularly blackleg and sclerotinia stem rot. For several years Monolit was a reference cultivar for testing other lines of oilseed rape at the Research Centre for Cultivar Testing (COBORU). As a result of including DHs in breeding programs in the Plant Breeding Company Smolice Ltd., another winter oilseed rape open-pollinated cultivar ‘Brendy’ (single DH line) has been bred in a shortened period of up to 5 years and was listed in the NLI in 2013.
Currently, DH technology is widely used to obtain homozygous lines for the creation of F1 hybrid cultivars in winter oilseed rape breeding. In Poland, the CMS ogura cytoplasmic male infertility system is used, and fertility is restored using the Rfo gene. Consequently, DH technology is primarily used for gene pool creation to restore male fertility (Popławska et al., 2007; Szała et al., 2016).
To broaden the genetic variation of oilseed rape, the diploid progenitor species Brassica rapa and Brassica oleracea were used through resynthesis of a new Brassica napus. Resynthesized (RS) oilseed rape is potentially of great interest for F1 hybrid cultivar breeding because the effect of heterosis is higher in crosses of genetically distant materials. However, RS lines are not suitable for direct use in breeding, mainly because of the low seed quality (Szała et al., 2016). Our strategy was to introduce double-low (00) quality traits into RS lines by crossing a 00-quality restorer line with RS oilseed rape, followed by in vitro ME of F1 hybrids. Subsequently, the desired and double-low DHs can be selected from the obtained population (Szała et al., 2016, 2019). Currently, 00-quality semi-RS DH lines are used for the development of F1 CMS ogura hybrids of winter oilseed rape. The results of the field experiments in 2020/2021 with such new F1 hybrids are very promising (unpublished data).
DHs have also been used in genetic marker development, QTL location, gene mapping, gene transformation, and other genetic studies, in which homozygous genotypes are required. Modern oilseed rape breeding relies on genetic molecular markers that significantly increase selection efficiency, particularly during the early stages of breeding. Using a DH segregating population of 250 DHs, the clubroot resistance locus in the winter oilseed rape cultivar Tosca was characterized (Kopec et al., 2021). Genetic mapping, structural genomics, expression analyses, and functional annotation led the authors to conclude that TNL gene (BnaA03g29300D) duplication is most likely involved in clubroot resistance. Based on these results, a functional marker will soon be developed for use in oilseed rape breeding and identified using DH lines. Genetic markers for low linoleic acid content (Mikolajczyk et al., 2010) and the restorer gene Rfo have already been implemented in breeding practice. For many years, PBAI-NRI research on the construction of a genetic map to identify QTL for erucic acid and glucosinolate content has been conducted based on molecular analyses (SNP, SSR) of over 100 DHs (Cegielska-Taras et al., 2015; Matuszczak et al., 2011).
Haploid structures are excellent materials for biotechnological manipulation and specific gene transfer, for example, by Agrobacterium tumefaciens-mediated delivery to haploid microspore embryos. A protocol for this has been developed in Tissue Cultures Laboratory of Department in Poznań (Cegielska-Taras & Pniewski, 2011; Cegielska-Taras et al., 2008).
The high effectiveness of our method in microspore-derived embryo (MDE) regeneration and in vivo chromosome number duplication allowed us to participate in innovative projects in which the transformation of microspore-derived embryos with A. tumefaciens was used as a research tool. The main goal of the first project was to apply ABI1 overexpression to plants to evaluate the pleiotropic effects caused by such a change in expression under drought conditions. In this study, we generated transgenic B. napus plants overexpressing the Arabidopsis thaliana ABI1 ortholog to study the corresponding changes in the drought stress response (Babula-Skowrońska et al., 2015). The second project concerned the conservation of the role of the ABI1 gene ortholog in Brassicaceae (B. napus vs. A. thaliana) and duplicated ABI1 genes in B. napus, with particular reference to the response to drought stress. DH transgenic B. napus plants overexpressing the A. thaliana ABI1 ortholog were generated to study the corresponding changes in the drought-stress response as well as the cumulative effect of all BnaABI1-like genes under drought conditions. The construct was introduced into B. napus microspore embryos via Agrobacterium-mediated transformation using the method described by Cegielska-Taras et al. (2008), and the plants were developed on selective media (Figure 7).
Figure 7
Regeneration of transgenic haploid winter oilseed rape. (A) Transformed microspore-derived embryos (MDEs) on a selection medium supplemented with kinetin. (B) Plantlet development from transformed MDEs on stable medium MS with kinetin and timentin. Photographs from the archives of the Department of Oilseed Crops, Plant Breeding and Acclimatization Institute – National Research Institute.
Currently, in oilseed rape breeding, much attention is paid not only to seed yield and oil content, but also to oil quality due to the increased nutritional value of oil for human consumption (Gacek et al., 2017). Our institute was involved in a 5-year program of the Polish Ministry of Agriculture for the enhancement of natural antioxidants and bioactive substances, such as tocochromanols, sterols, and phenolic compounds, in oilseed rape. These studies were realized with the use of different populations of DH lines specially obtained for this program (Cegielska-Taras et al., 2016; Siger et al., 2015, 2018).
Oilseed rape seed meal is an important source of protein, but the presence of anti-nutritional compounds, such as fiber and glucosinolates (GLS), still limits its use as livestock feed. The population of DH lines developed from F1 hybrids obtained by crossing DH M305 black seed × DH Z114 yellow seed has been used for analyses of genetic variation of traits affecting the byproduct of oil production from this crop, a protein rich in oilseed rape meal. The results suggest a large genetic variation in these traits and the interrelationships among them. The analysis of heritability and gene effects on the studied traits allowed the estimation of the genetic background of these traits (Wolko et al., 2020). The same population (DH M305 × DH Z114) of 78 DHs was used in the QTL mapping analysis to explain the genetic basis of the characteristics affecting the quality of oilseed rape meal: protein, fiber, GLS, and seed coat color. The aim of this study was to identify SNPs significantly associated with interesting traits to determine candidate genes and develop genetic markers that could be used in breeding programs to improve the quality of oilseed meal (Gacek et al., 2021).
. DH Production in Oat (Avena sativa L.) Through Distant Crossing
Obtaining oat DH lines is difficult, and the effectiveness of the technique highly depends on the methods used and the genotype of the donor plants. Literature data and research conducted at the Franciszek Górski IPP PAS show that oat DH can be obtained mainly by distant crossing via pollination with maize. However, this method still requires optimization of the culture conditions, mainly during the germination of haploid embryos, plant regeneration, and acclimatization to ex vitro conditions, as well as restoring fertility (doubling the number of chromosomes).
The first experiments based on chromosome elimination were devoted to improving the effectiveness of haploid collection and focused on several aspects of this process: (i) genotype of donor plants, (ii) selection of a pollen donor plant, (iii) time between emasculation and pollination, (iv) influence of auxin on ovary enlargement and embryo production, (v) time between pollination and application of growth regulators, and (vi) type of regeneration medium on embryo germination and plant growth. The strong influence of the genotype was determined by Sidhu et al. (2006) and confirmed by Marcińska et al. (2013), showing a large spread of values from 0.2% to 2.6% DHs per pollinated floret. For haploid oat production, maize (Zea mays var. saccharata) pollen is usually used, with pearl millet (Pennisetum glaucum L.), common millet (Panicum miliaceum L.), and sorghum [Sorghum bicolor (L.) Moench] (Nowakowska et al., 2015) being less often used. The time between emasculation and pollination seemed to significantly influence embryo production, regardless of the oat genotype. According to Marcińska et al. (2013), pollination one and 2 days after emasculation resulted in the production of 5.7 and 13 embryos per 100 florets, respectively. In addition, the influence of different growth regulators [picloram, dicamba, gibberellic acid (GA3), and 2,4-dichlorophenoxyacetic acid (2,4-D)] was tested for their capacity to induce caryopsis (Figure 8A) and embryo formation (Figure 8B), but no statistically significant differences between applied substances or significant Growth Regulator × Genotype interactions were observed. However, Marcińska et al. (2013) noticed that although two applied auxins, dicamba and 2,4-D, generated a similar number of enlarged ovaries and embryos, dicamba treatment resulted in enlargement of ovary size compared to 2,4-D. Nevertheless, 2,4-D turned out to be more effective in converting embryos to haploid plants (1.4%) (Figure 8C) as well as obtaining DH lines (0.5%), whereas dicamba treatment resulted in 0.6% of haploid plants and 0.3% of DH lines (Figure 8E) (Warchoł et al., 2016).
Figure 8
Oat (Avena sativa L.) doubled haploids (DH) production by distant crossing with maize (Zea mays var. saccharata). (A) Caryopses after 100 mg L−1 2,4-D treatment. (B) Haploid embryo isolated from ovary. (C) Germinated haploid embryo on 190-2 medium. (D) Oat chromosomes with tetrasomic addition of maize chromosomes. Multicolor-genomic in situ hybridization: Maize genomic DNA labeled with biotin-16-dUTP and detected with streptavidin-Cy3 (red), 45S rDNA labeled with digoxigenin-11-dUTP and detected with anti-digoxigenin fluorescein isothiocyanate (FITC, green) and telomeric repeat sequences directly labeled with Cy5 (magenta). Chromosomes counterstained with DAPI (blue). (E) Maturing DH plants in the greenhouse.
The highest number of embryos were obtained with the application of growth regulators starting at 2 days after pollination (10.4 embryos per 100 florets). Extending this time to 3 or 5 days decreased the number of embryos obtained to 4.8 and 6.2 per 100 florets, respectively (Marcińska et al., 2013). As haploid oat embryos develop without an endosperm, but in co-culture with ovaries, the changes in the content of phytohormones in ovaries during the development of haploid embryos were measured (Dziurka et al., 2019). Significantly higher concentrations of indolyl-3-acetic acid (IAA), trans-zeatin, and kinetin (KIN) were found in ovaries with embryos than in those without embryos. Lower concentrations of KIN in ovaries increased the efficiency of haploid plant production. The presence of 4-chloroindole-3-acetic acid, a hormone that has been proposed as an aging factor in plants, was confirmed in ovaries without embryos. Because the endosperm usually fails to develop, isolated haploid embryos must be cultivated in regenerating medium. According to Marcińska et al. (2013), the 190-2 medium (Zhuang & Xu, 1983) was better than TL3 (Taira & Larter, 1978), with 41.2% versus 23.6% regenerated embryos. Finally, 10 haploid plants (19.6%) on 190-2 and four haploid plants (5.6%) were regenerated on the TL3. The most effective germination of haploid embryos was observed in 190-2 medium with 9% maltose and pH 6.0 (9.1%) (Warchoł et al., 2018). Moreover, most haploid embryos germinated on 190-2 medium with addition of 0.5 mg L−1 NAA and 0.5 mg L−1 KIN (Noga et al., 2016). It has also been shown that light intensity during in vitro culture can significantly affect the development of haploid embryos. A light intensity of 110 µmol m−2 s−1 resulted in the highest percentage of embryo germination (38.9%), conversion into plants (36.4%), and DH line production (9.2%) when compared with lower light intensities (20, 40, and 70 µmol m−2 s−1) (Skrzypek et al., 2016). Chromosome doubling is an essential step in oat DH line production. Treatment of haploid plants has been successfully performed using 0.1% colchicine solution (Marcińska et al., 2013; Noga et al., 2016; Nowakowska et al., 2015). The most efficient method for oat DH production through wide hybridization with maize has been described in detail by Skrzypek et al. (2021).
In distant crosses within a specific Poaceae subfamily, for example, oat × maize, male chromosomes are preferentially eliminated during early embryo development, although the elimination of chromosomes may be incomplete. As a result, a hybrid zygote is formed, and subsequently, an embryo and stable hybrid plants that retain the single maize chromosomes are produced (Skrzypek et al., 2018). Among 138 oat lines obtained, the presence of maize chromatin was confirmed in 66 lines (48%) by amplification of a PCR product (500 bp) produced with primers specific for Grande-1 maize retrotransposons. Cytogenetic analysis using genomic in situ hybridization confirmed the presence of whole maize chromosomes in eight lines and the insertion of maize chromosome fragments in 20 lines. The obtained oat × maize addition (OMA) lines contained one to four maize chromosomes (Figure 8D). At the same time, all the lines tested had a complete set of oat chromosomes. More precise studies have been undertaken to visualize the nuclear architecture of interspecies hybrids (Idziak-Helmcke et al., 2020) as it may affect their genetic stability and usefulness in the effective selection of traits desired in agriculture. In the hybrid lines, the territories of maize chromosomes were observed to resemble rather compact surfaces, indicating that they do not follow the typical oat Rabl configuration distinguished by the clustering of centromeres on one side of the nuclear envelope. Retention of the maize chromosome(s) (C4 plant), in addition to morphological and physiological aberrations, may also affect the functioning of the photosynthetic apparatus and increase the tolerance of the hybrids to drought stress. The changes observed in the functioning of photosynthetic apparatus of hybrids depend more on the particular maize chromosomes retained and their interaction with the oat genome rather than on the number of preserved chromosomes alone (Juzoń et al., 2020).
Many years of research conducted at the IPP PAS in cooperation with Polish breeding companies (DANKO Hodowla Roślin Sp z o.o.; Hodowla Roślin Strzelce Sp. z o.o. IHAR Group and Małopolska Hodowla Roślin Sp z o.o.) have resulted in the introduction of about 500 oat DH lines to the breeding programs. In 2020, DANKO Hodowla Roślin Sp z o.o. registered a new oat cultivar called ‘Huzar,’ derived by DH technology in IPP PAS.
. DH in Sugar Beet (Beta vulgaris L.): An Example of the Application of Gynogenesis to Plant Breeding
Sugar beet (Beta vulgaris L.) is one of the most important industrial crops cultivated mostly in zones of temperate climate (i.e., Central and Southern Europe, USA, etc.). This crop ranks as the second largest source of sugar worldwide, accounting for approximately 20% of world sugar production, and the largest source in Europe (Eurostat & Cook, 2020). It is also used to produce a wide range of products, including feed, bio-based products (pharmaceuticals, plastics, textiles, and chemicals), and ethanol (Organization for Economic Co-operation Development & Food and Agriculture Organization, 2020).
Since the nineteenth century, outstanding progress has been made in sugar beet breeding. The technical progress, supported by attention to factors such as agronomy, climate, and conventional and biotechnological breeding achievements, contributed to noticeable taproot yield improvement, where the sugar content had been increased from 8% to 18% in modern cultivars (Dohm et al., 2014; Stevanato et al., 2019). The main conventional breeding strategy is based on the utilization of heterosis through efficient production of F1 hybrids using monogerm male sterile lines crossed with multigerm pollinators (Gośka, 1999). Heterosis can be achieved through crossing experiments to identify parental germplasm pools (Hallahan et al., 2018). Due to the biennial life cycle, allogamy, self-incompatibility, and inbreeding depression, the traditional production of inbred lines developed from heterozygous plant material requires time-consuming and labor-intensive backcrosses (Zhuzhzhalova et al., 2020). It takes at least three generations, which requires 6–7 years.
Attempts to induce haploids using in vivo methods began in 1943, but they did not yield useful efficiency (Levan, 1945). As an alternative, the use of haploid and DH in vitro technology enables the creation of numerous completely homozygous lines from heterozygotes in a single generation (Sohrabi et al., 2021). To increase competitiveness, breeding companies must be able to produce new cultivars within the shortest period. In recent years, old cultivars have been rapidly replaced with new ones. Competition between breeding companies leads to the situation that a cultivar that has been on the market for 4–5 years is considered old, which necessitates the annual registration of new ones.
Research on obtaining sugar beet haploid and DH lines has been carried out at the Laboratory of Cytogenetics and Breeding Methods in Plant Breeding and Acclimatization Institute – National Research Institute (PBAI-NRI) in Bydgoszcz Division since the end of the 1970s. Because in many agronomically important crops the most popular and successful DH technology is based on anther or isolated microspore in vitro culture, attempts at ME were made. However, only calluses and roots were recovered, or the efficiency of plant regeneration was at a very low level (0.02%), and the cytological analyses indicated diploids, which suggested a somatic source for the recovered plants. Sugar beets were therefore considered recalcitrant to ME (Gośka, 1986; Rogozińska & Gośka, 1976). A breakthrough came in the early 1980s with the production of gynogenic haploids using in vitro culturing of ovaries or ovules, first reported in sugar beet by Hosemans and Bossoutrot (1983), and fully accomplished in 1985 (Gośka, 1985).
In the following years, research efforts by Gośka (1997) have resulted in further progress in sugar beet gynogenesis. An efficient protocol for DH production from ovules was established and implemented in breeding practices in the Kutnowska Hodowla Buraka Cukrowego Sp. z o.o. (KHBC Sp. z o.o.). The methodology includes several steps. The first was the isolation of ovules and regeneration of haploid plants. Genotype dependency is one of the most important factors affecting the efficiency of the entire process. Cultivars of the same species have different gynogenetic capacities, conferring the strongest limitation on the process, whereas other environmental factors such as donor plant growth conditions, flower bud morphophysiology, medium composition, and culture conditions can be optimized. The best gynogenesis response was obtained when isolating the ovules from the closed floral buds above the floral bud in the anthesis stage. The optimal medium for embryogenesis is based on Murashige and Skoog medium (MS) (1962) supplemented with 0.3 or 1.0 mg L−1 BAP and 0.1 mg L−1 NAA. Depending on the genotype of the donor plants, the use of appropriate external conditions during the embryogenesis induction stage for in vitro cultures enabled recovery of 31% haploids. It has been confirmed that haploid embryos originate from unfertilized egg cells or, less frequently, from synergids. The earliest stages of haploid embryo development in vitro (until the globular phase) are similar to those observed during in vivo embryo formation. Such direct embryogenesis without the callus phase is beneficial in offering greater consistency. Plants obtained in this way have a specific genotype, while plants obtained from callus organogenesis have different ploidy levels with the possibility of somaclonal variation.
Because of meiotic abnormalities, which cause microsporogenesis disorders, haploids were infertile. Spontaneous diploidization occurs in 10% of haploids, hence the number of chromosomes needs to be doubled with an anti-mitotic agent such as colchicine. The highest efficiency of diploidization (50%–100%) was achieved with the double treatment of haploid floral buds with a solution of 0.65% colchicine along with a single application of 1.3% colchicine. Based on morphological features, DH plants from the same genotype showed intra-line stability, whereas diversity between different lines was observed, especially in the second vegetative season. DH lines set a small number of seeds, at the level of 1.1%–20.1% depending on the genotype. Out of 109 DH lines, 29 produced seeds (26.6%). The limited number of seeds and low seed vigor were caused by self-incompatibility and semi-lethal gene expression in the homozygous configuration (Figure 9).
Figure 9
Gynogenesis in unfertilized ovule culture of sugar beet (Beta vulgaris L.). (A) Donor plant during field cultivation. (B) Floral buds suitable for ovule isolation. (C) The ovule and the ovary. (D) Morphology of embryo sac with the unfertilized egg cell before division. (E) The ovules on induction medium. (F) Haploid plantlets on regeneration medium. (G) Doubled haploids, 2 months after colchicine treatment and acclimatization to ex vitro conditions.
Further studies conducted in IHAR-PIB, financially supported by the Polish Ministry of Agriculture and Rural Development, enabled the biological and molecular characterization of haploids, DHs, and donor plants. For this purpose, both ISSR and RAPD marker systems were used to analyze 30 maternal lines, 76 haploids, and 54 DHs (M. Gośka, personal communication, 2016). The unique band pattern characteristics of each tested genotype were determined. Among the analyzed loci, we observed genetic differences between haploid lines recovered from the same heterozygous maternal genotype but different ovules. This is the same as in the case of DH lines. However, among haploids and DHs recovered from the same ovary, genetic similarity was very high, suggesting that little or no variation resulted from the use of the anti-mitotic agent.
Despite the significant progress in gynogenesis, attempts have been made to further increase the efficiency of this process. Currently, little is known about the cytological or molecular mechanisms such as gene expression involved in the induction of gynogenesis. In another study carried out at the IHAR-PIB, the participation of arabinogalactan proteins (AGPs), known cell wall remodeling agents, in the development and differentiation of cells and tissues during sugar beet growth in in vitro cultures has been demonstrated (Wiśniewska & Majewska-Sawka, 2007, 2008). The addition of exogenous 2.5 ug mL−1 AGPs to the liquid medium at the beginning of the guard protoplasts stage had an important influence on callus organogenesis induction. The effectiveness of the entire process increased from 6.8% (control) to 47.2% (AGP addition). AGPs showing a positive biological effect were found to be rich in oligosaccharide epitopes recognized by the JIM13, MAC207, and LM2 antibodies. These results suggest that AGPs play an important role in the development of sugar beet guard cell protoplasts and the organogenesis of protoplast-derived calli. Therefore, it is advisable to analyze the presence and distribution of AGPs and pectin structural motifs in ovules during in vitro culture. Detailed examination of cell walls deposited by ovules during in vitro culture revealed the presence and widespread distribution of AGP epitopes, such as LM2, JIM13, and JIM8. Additional characteristics of these epitopes are yet to be determined.
. Haploids and DHs in Vegetable Crops
At the Department of Plant Biology and Biotechnology (formerly Department of Genetics, Plant Breeding and Seed Science) of the University of Agriculture in Krakow (URK), research on gametic embryogenesis was initiated in 1990 by Prof. Barbara Michalik and co-workers. Early studies aimed at obtaining haploids in red beets (Beta vulgaris L.) and focused on the evaluation of the factors influencing the development of ovules isolated in vitro. These studies showed that the gynogenic response depended mostly on the genotype, but also on the season, growth conditions, and composition of the medium. Ovules mainly developed in deep-red calli, radicles, and cotyledons (Barański, 1996) and only single plants were obtained. Further research focused on the ability of sugar beet haploid and DH materials to micropropagate and their stability at the ploidy level. Although beet plantlets are sensitive to culture conditions, haploids often perform better than DHs, as manifested by a 2-fold higher frequency of petiole and midrib explant regeneration and a 3-fold higher regeneration rate (Klimek-Chodacka & Baranski, 2013). Spontaneous ploidy changes were observed in prolonged in vitro culture of haploid plantlets. After 2–4 year of culture, most of the micropropagated plantlets were diploid or mixoploid. In three out of nine tested clones, approximately half of the micropropagated plantlets were still haploid, and only one clone retained its original haploid status (Klimek-Chodacka & Baranski, 2011). These results indicate the potential of haploid beet materials for future improvements using novel gene engineering techniques.
In carrot (Daucus carota L.), hybrid cultivars are produced using inbred populations obtained after several generations of self- or sib-pollination, resulting in strong inbreeding depression. Studies of gametic embryogenesis in this species have been conducted since 1999. Anther culture has proven to be a poor haploidization technique because of the high rate of callogenesis and somatic embryogenesis from the anther walls (Adamus & Michalik, 2003). Therefore, we developed a protocol for the induction of carrot haploid plants using female gametophytes (Kiełkowska & Adamus, 2010). Our study showed that the development of unfertilized carrot ovules was possible after pollination with foreign pollen. Parsley pollen was found to be the most suitable. Most plants obtained using our protocol were haploids and diploids derived from parthenogenesis, as evidenced by homozygosity at three independent loci based on isozyme and PCR analyses (Kiełkowska et al., 2014, 2018).
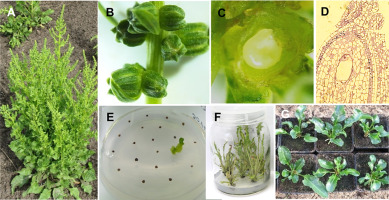
Research on gametic embryogenesis has also been conducted for different members of the Brassicaceae family. ME was induced in anther cultures and later in cultures of isolated white cabbage microspores (Brassica oleracea var. capitata) (Figure 10A–D), brussel sprouts (B. oleracea var. gemmifera), cauliflower (B. oleracea var. italica), and radish (Raphanus sativus) (Adamus, 1993, 1994, 1998; Michalik et al., 2001). The results of the induction of embryogenesis in the cultures of isolated microspores showed that Polish breeding materials of cabbage (more than 50 different accessions) had a rather low ability (none or a single embryo per 100 flower buds) to undergo gametic embryogenesis (Adamus, 2001; Adamus & Samek, 2006; Adamus et al., 2002). In addition, the low survival rate of the embryos obtained is a problem. Application of a desiccation procedure to the embryos obtained using abscisic acid (ABA) significantly improved the conversion of embryos into plants (Figure 10E,F) (Adamus et al., 2002). Regardless of the problems at the culture stage, we were able to obtain DH plants for all species tested, but the highest success rate was obtained for cabbage. The DH cabbage plants were evaluated according to their homozygosity, ploidy, trait uniformity (Figure 10G,H), and fertility (Adamus, 1998; Adamus & Samek, 2006; Barański, 2000; Combik et al., 2006). Next, DH plants were subjected to self-pollination to obtain DH lines, which were further evaluated for their antioxidant properties (Leja et al., 2006). Twenty-five DH lines were examined and compared with the standards (two F1 hybrids) and parental genotypes. Significant differences in ascorbic acid and phenolic content were detected among the tested DH lines. Some DH lines showed a high content of ascorbic acid and soluble phenolics, which was also accompanied by high antiradical activity. These studies showed that with the use of protocols optimized in our laboratory, it is possible to obtain and select valuable DH plants in cabbage. Recently, ME was also induced in Brassica rapa L. subsp. pekinensis (Adamus et al., 2021). Using our protocol, DHs and later DH lines were obtained and released to a Polish breeding company for further evaluation (Adamus et al., 2017, 2018).
Figure 10
Androgenesis in microspore culture of Brassica oleracea var. capitata L. (A) Inflorescence of cabbage, buds suitable for androgenesis marked with an arrow. (B) First mitosis in isolated microspore culture marked with arrows. (C) Androgenic embryos. (D) Regenerated plantlet. (E,F) DH plants after acclimatization. (G,H) DH lines in the field experiment.
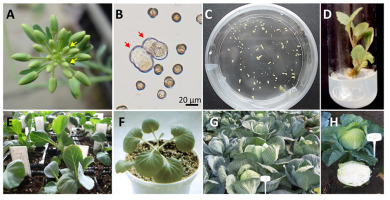
Research on gynogenesis in onion (Allium cepa L.) has been conducted since 1995 in response to the need to shorten the breeding process of obligatory biennial crop species. In vitro culture of whole flower buds (Figure 11A–C) gave positive results (Michalik et al., 1997). Further studies have shown that the effectiveness of gynogenesis in onion is highly dependent on the genotype and media composition (Michalik et al., 2000). After determining the optimal parameters (flower bud size, medium, light conditions, and regeneration conditions), haploid embryos were obtained (Figure 11D,E). A major achievement was the development of a protocol for diploidization of gynogenic embryos. This is a very important issue, as haploids are sterile, and spontaneous diploidization is rare in onions (Bohanec, 2002). The utilization of haploids for breeding depends on effective doubling of the genome. Genome doubling was induced in vitro using four anti-mitotic agents, trifluralin, oryzalin, amiprophos-methyl (APM), or colchicine at various concentrations. The highest doubling efficiency, combined with the lowest side effects (toxicity and vitrification), was achieved for embryos cultured on media supplemented with APM (Grzebelus & Adamus, 2004). The DH lines obtained were also assessed for their suitability for hybrid breeding (Figure 11F,G) (Adamus et al., 2005).
Figure 11
Gynogenesis in whole flower bud culture of Allium cepa L. (A) Inflorescence of onion with buds suitable for gynogenesis. (B,C) Gynogenic embryos (arrows) emerging from enlarged greenish or parchment ovaries plated on solid media. (D) Plantlet regenerated from embryos. (E) DH plant after acclimatization. (F,G) DH lines in the field experiment and bulb evaluation.
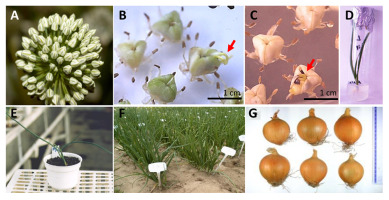
The Department of Plant Biology and Biotechnology, URK, has cooperated with Polish breeding companies (PlantiCo, KHiNO POLAN, and Spójnia Nochowo) for over 30 years. Cooperation has focused on using modern biotechnological methods to support breeding programs for vegetable crops. This research resulted in the development of innovative methodologies for obtaining DH plants by ME for cabbage and gynogenesis in the cultures of flower buds for onion. These methodologies were improved in the following years. The resulting protocols for the induction of DHs in cabbage and onion, as well as the plant materials obtained in this study, were released for the breeding programs of these companies.
As a result of implementing the methodologies for haploid induction developed at URK and based on the plant materials provided, breeding companies developed new varieties of cabbage and onions, which were registered by the Research Center for Cultivar Testing (COBORU) in Słupia Wielka, Poland. The register of COBORU included two hybrid cultivars of onion: Warsa F1 (entry 2009, PlantiCo Zielonki) and Wega F1 (entry 2017, Spójnia Nochowo), and three varieties of cabbage bred by KHiNO POLAN: Korund F1 (entry 2012), Jasper F1 (entry 2017), and Opalo F1 (entry 2017). Following this registration, the seeds of these cultivars were introduced to the national market for both professionals and amateurs. Moreover, in pre-registration field experiments carried out in COBORU, another hybrid cabbage cultivar (POLB1 – KHiNO POLAN) was developed based on our protocols. This indicates that the methods developed are useful for breeding new cultivars.
All mentioned cultivars were also included in Katalog odmian warzyw kwiatów i ziół [Catalogue of cultivars of vegetables, flowers, and herbs], and therefore are available to producers of cabbage and onions on the European market. It is worth mentioning that cabbage varieties Jasper F1 and Korund F1 are characterized by very valuable features, such as tolerance to thrips (Thrips sp.), a dangerous pest with enormous reproductive ability, serving as a vector of many viral plant diseases, and bacterial and fungal diseases, in particular to alternariose (Alternaria brassicae) and downy mildew caused by Hyalperonospora parasitica (Catalogue of Cultivars of Vegetables, Flowers, and Herbs, 2020, pp. 126–127). Therefore, these cultivars are recommended for use in organic farming (Rogacz, 2019; Żołnierkiewicz, 2019), as their production can be carried out with the limited use of agrochemicals, which is crucial for production of health-promoting foods and for protection of the environment.
. Final Remarks
Research by Polish scientists has made important contributions to our understanding of the complex mechanisms of diverse processes leading to doubled haploid (DH) plant production (microspore embryogenesis, gynogenesis, and distant hybridization followed by genome elimination). The studies have resulted in the following conclusions.
Knowledge of the mechanisms of microspore reprogramming and redirection toward embryogenic development in triticale. This revealed the role of ROS as signaling molecules involved in ME induction and the significant effect of antioxidative defense efficiency on ME effectiveness. Together with data obtained from (epi)genetic, hormonal, and proteomic analyses, it has provided the basis for some practical improvement of in vitro culture protocols for other, more recalcitrant crops (e.g., rye).
Insights into the genotype-dependent molecular processes underlying the formation of microspore-derived albino regenerants. The lack of PEP RNA polymerase activity in plastids is associated with impaired chloroplast differentiation during plant regeneration. It has been suggested that the insufficient number of complete plastome copies might be a retrograde signal that hampers correct plastid biogenesis and chloroplast differentiation in albino-producing genotypes. This knowledge helps overcome the problem of albinism in the androgenic culture of barley and other cereals.
Development of (i) methods of isolated microspore culture for obtaining many DH lines of oilseed rape, triticale, barley, and rye, and (ii) the most efficient method of oat DH production through wide hybridization with maize. Moreover, the incomplete elimination of maize chromosomes resulted in the formation of stable oat-maize hybrid plants. The obtained OMA lines showed morphological and physiological aberrations, which affected the functioning of the photosynthetic apparatus and could possibly increase the tolerance of the hybrids to drought stress; (iii) an efficient protocol for sugar beet DH production from ovules; and (iv) methods for DH production for several vegetable crops such as red beet, onion, carrot, and some Brassicaceae species.
The implementation of the studies described has provided an invaluable tool to shorten breeding cycles, improve breeding efficiency, and increase genetic gain. It is possible to produce fully homozygous lines in one generation, accelerate the development of cultivars with desired market traits, and obtain excellent haploid plant material for basic research in areas such as molecular physiology, genomics, gene expression, and genetic mapping.


