The paper is dedicated by J. J. Rybczyński (President of Society 2004–2010) and A. Mikuła (President-in-charge) to the celebration of the 100-year anniversary of the Polish Botanical Society foundation.
. Introduction
The majority of tree ferns with prominent trunks constitute two families: Cyatheaceae and Dicksoniaceae (Moura et al., 2012); however, there are at least four families that include short trunk ferns. Intensive growth of tree ferns results not only in huge body formation but also in intensive metabolite production (Banerjee & Sen, 1980; Bharti, 2018). The horticultural industry of tree ferns depends greatly on the species (Das et al., 2013). In the case of Cyathea smithii, there is no scientific information in the experimental botany references contrary to scientific progress described for C. australis (Goller & Rybczyński, 1995, 2007) and C. delgadii (Mikuła et al., 2015).
General Characteristic of Cyathea smithii
The plant used in this study was C. smithii. It is a medium-sized tree fern that can produce a slender trunk up to 8 m tall but is usually considerably shorter. It has a crown of flat, tripinnate, and profusely scaly leafstalks. Stalks persist on the trunk and can form a skirt below the crown. C. smithii is native to cool mountain and lowland forests on the North and South Islands of New Zealand, as well as Stewart Island, the Chatham Islands, and the sub-Antarctic Auckland Islands far to the south, making it the world’s most southerly tree fern. It is well adapted to the cool and humid conditions of its habitat, grows poorly in heat, and is less tolerant to higher temperatures than other tree ferns from comparable habitats, such as Dicksonia antarctica. Cyathea smithii tolerates moderate frost and is one of the hardiest tree ferns, although it is not as hardy as D. antarctica or D. fibrosa (Jones, 1987; Large & Braggins, 2004).
Gametophyte Axenic Culture
Gametophytes are the most popular generation for tree fern generative propagation, initiated by spore germination and prothallus culture in vitro (Das et al., 2013; Shukla & Khare, 2012; Takahashi et al., 2015). Fern gametophytes have three distinct meristems: apical cell-based, marginal, and multicellular meristems (Imaichi, 2013). The meristematic activity of marginal meristem has been studied for gametophyte multiplication and the formation of deep green clumps (Goller & Rybczyński, 1995). There are numerous examples of unlimited fern multiplication that depend on medium richness and period of subculture (Goller & Rybczyński, 2007). The period of subculture is determined by the size of “explants” used for its initiation and the volume of the medium. In a closed container, increasing humidity and water condensation on the surfaces of cultured gametophytes may result in numerous spontaneous apogamy occurrences, giving one sporophyte per gametophyte depending on the species cultured.
Sporophyte Culture and Plant Growth Regulators
Hormone-free somatic embryogenesis induction of C. delgadii requires a different approach for tissue culture and cell manipulation of ferns (Mikuła et al., 2015). Previously, various plant growth hormones were applied for vegetative propagation [most often 6-benzylaminopurine (BAP) and naphthalene acetic acid (NAA) (Avila-Pérez et al., 2011; Nakamura & Maeda, 1995; Shukla & Khare, 2012) and 2-isopentinyladenine (2iP) (Liao & Wu, 2011)] but for callus induction and proliferation, 2,4-D (Shukla & Khare, 2012) was used. 2,4-D is an artificial plant growth regulator with biological activity similar to that of natural auxins (at low concentrations) and has been studied since the middle of the twentieth century (Tukey, 1947). Other auxins like indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) and NAA were used to control the apogamous sporophyte development of C. spinulosa with best results achieved on the level of three leaves and two roots, with 1.0 mg/L IAA supplementing Knop’s basal medium (Parajuli & Joshi, 2014). Limited results in vegetative propagation have led us to consider thidiazuron [TDZ; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea)], a urine-derived plant growth regulator with potential activities for shoot regeneration and shoot proliferation. In general, its biological activity induces cytokinin activity or accumulation of endogenous cytokinins. In the case of ferns, TDZ is rarely applied and has only been used for green globular body induction, and its development has resulted in the regeneration of Cibotium barometz. Experiments have been conducted on explants originating from juvenile sporophytes in vitro (Yu et al., 2017). TDZ is very important for the vegetative propagation of recalcitrant woody plants. Previous studies have shown that TDZ can induce somatic embryogenesis in both woody and green plants. Lupinus albus, an annual green plant, is a very good example of somatic embryogenesis induction in the culture of cotyledons of immature embryos isolated from green pods (Rybczyński et al., 1999). Two different responses to TDZ treatment have been reported for Lens culinaris (Chhabra et al., 2008). TDZ was the only plant growth regulator (PGR) used in this study. Lens culinaris cotyledonary node explants responded to TDZ treatment by forming shoots or somatic embryos at a critical concentration of 2.0 µM. Concentration of TDZ lower than 2.0 µM stimulated shoot organogenesis, whereas higher concentrations up to 20.0 µM stimulated somatic embryogenesis (Chhabra et al., 2008). In the monocot Saccharum officinarum, somatic embryogenesis is induced by TDZ medium supplemented with 2,4-D (Malabadi et al., 2011).
Gametophyte and Sporophyte in Relation to Vegetative Propagation of Tree Ferns
Most studies on the vegetative propagation of tree ferns describe the establishment of gametophyte multiplication cultures on the basis of spore germination. Studies have discussed how to develop improved methods to produce unlimited numbers of sporophytes through apogamy (Goller & Rybczyński, 2007; Kuriyama et al., 2004; Rybczyński et al., 2018). Previous research has allowed for the production of unlimited numbers of individuals as the sources of the explants of apical meristems, young leaves, and roots for fern research. However, this research rarely included tree ferns since the first paper describing the spore germination patterns of C. australis, C. cooperi, and D. antarctica was published by Huckaby and Raghavan (1981). Later spore culture was employed for vegetative propagation of C. australis and next 16 species of tree ferns (Goller & Rybczyński, 1995, 2007). These studies did not include plant growth hormones, and gametophyte propagation was performed in a hormone-free medium. Under PGR treatment, the transition of gametophytic meristems (apical cell, cell-based meristem, and marginal meristem) into shoot apical meristem could result in significant progress in fern morphogenesis. The comparison of two generations with different ploidy levels of the same genome would help illustrate generational differences at the molecular level.
Among the various methods of biological evaluation of regenerants, the chromosome number and later employed nuclear DNA content have been used (Doležel & Bartoš, 2005; Galbraith et al., 1997) extensively for the selection of new individuals in breeding programs. Both methods are being increasingly employed to determine the genetic value of ferns (Bennett & Leitch, 2001). Obermayer et al. (2002) published an analysis of nuclear DNA C-values in 30 species and doubled the number of studied families of Pterydophytes, and the next 31 species of Monilophyta were the objective of DNA content variation studies (Bainard et al., 2011). Among the tree ferns of Cyathea genus only C. crinite and C. australis were listed with following data of the nuclear DNA 1C = 7.4 and 4.91 pg and 2C = 14.7 and 9.92 pg, respectively (Mikuła et al., 2009; Obermayer et al., 2002).
The growth of two free-living generations of ferns, gametophytes and sporophytes, makes this plant material suitable for experimental biology and biotechnology research, with the best example being the aquatic fern Ceratopteris richardii (Johnson & Renzaglia, 2008; Salmi et al., 2005). Both generations have a limited lifespan and meristematic activity. Present tools of biotechnology make plants excellent experimental materials, and the above-mentioned limitations do not exist since in vitro experiments started in the middle of the last century. The exceptionality of this phenomenon lies in the existence of one genome with two morphologically and physiologically different forms, i.e., generations, with unlimited access to them.
In the genus Cyathea, C. smithii is interesting because it has apical meristem activity without the potential for adventitious bud formation or any other type of vegetative propagules in nature. The apical dome meristem is involved in trunk (erected rhizome) elongation and three-leaf primordia formation in a whorl from the apical meristem controlled by tetrahedral apical initial cells (Ambrose & Vasco, 2016).
An aim of this study was to determine whether the apical dome is an appropriate explant for studying the morphogenic potential of sporophyte generation in vitro. For the uniform description of the regenerants, in addition to their morphological characteristics, nuclear DNA content was used. The obtained results allow us to discuss the following aspects: first, the morphogenic potential of the apical dome meristem of sporophytes (White, 1979) with special attention paid to the role of tetrahedral cells and apical meristem, and second, the total nuclear DNA content of its regenerants.
. Material and Methods
Medium Description
In all the experiments, the plant material was cultured in 1/2 Murashige and Skoog (MS) mineral medium (Murashige & Skoog, 1962). Supplementation with plant growth regulators was changed according to the needs of the experiment. Sucrose 2% was the only source of organic carbon (Sigma-Aldrich). Before sterilization by autoclaving, the medium was adjusted to pH = 5.8 and to pH = 5.32 after autoclaving. Its conductivity was adjusted to 97.0 mV (H2O double-distilled pH = 5.73, conductivity 74.4 mV). The medium was solidified with agar (Duchefa) at 8 g/1 L.
Plant Material Origin
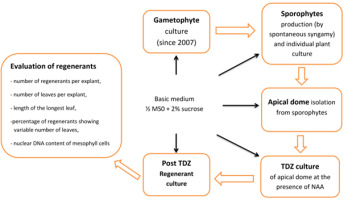
Plant material originated from a long-term axenic gametophyte culture of Cyathea smithii Hook. f. (soft tree fern, Katote) maintained on hormone-free 1/2 MS medium supplemented with 2% sucrose (Goller & Rybczyński, 2007). Sporophytes were collected after to 3–6 months. Plant production is initiated by a single gametophyte. Spontaneous, uncontrolled syngamy of C. smithii was the main source of plant formation according to biological principles: one gametophyte, one fertilized egg cell in one archegonium, and one sporophyte. To determine the nuclear DNA content of regenerants, gametophytes originating from standard culture were used as the haploid control, and sporophytes cultured out of TDZ treatment were used as the diploid control (Figure 1).
TDZ Effect on the Meristem Cell Multiplication and Plant Regeneration
Experiment was carried out using two steps of culture of explant; the first phase consisted of plant material culture in the presence of four concentrations of TDZ (0.01, 0.1, 1.0, and 5.0 µM + control) supplemented with 0.25 µM NAA, and second phase consisted of stimulation of the overgrowth of multiplied meristems maintained on 1/2 MS hormone-free medium for shoot development and plantlet regeneration. The control culture was maintained in a hormone-free medium throughout the experiment. Fifty explants were cultured for each TDZ concentration (2 × 25 apical domes). To evaluate the morphogenic potential of explants, four factors were considered: (i) the number of regenerants per individual apical meristem, (ii) the number of leaves per individual regenerant, (iii) the length of the longest leaf per regenerant, and (iv) the nuclear DNA content in green leaf mesophyll cells of leaf blade tissue.
Evaluation of Nuclear DNA Content
Samples were taken from three to five leaves collected randomly from one sporophyte plant or gametophyte clump of each genotype or line cultured in vitro. Leaf tissue (15 mg) was chopped together with 15 mg of young leaf maize (Zea mays L.) CE-777 2C DNA = 5.43 pg (Lysak & Doležel, 1998) as the internal standard in a Petri dish in 1.5 mL nuclei isolation Partec buffer (Sysmex Partec GmbH, Görlitz, Germany) with propidium iodide (50 µg/mL), RNAse (50 µg/mL), and 1% polyvinylpyrrolidone (PVP). Maize seeds of the reference plants were kindly provided by the Institute of Experimental Botany, Czech Republic. The chopped samples were filtered through a 30 µm filter and incubated for 60–90 min at room temperature. Nuclei fluorescence was measured using a CyFlow Ploidy analyzer (Partec, Germany). The 2C DNA content of each sample was calculated as the sample peak mean divided by the mean of the maize peak (internal standard) and multiplied by the maize 2C nuclear DNA content. The DNA content of three–five leaves of each plant with a minimum of 2,000 nuclei was measured, with two runs from each nuclei isolation extract. Flow cytometry studies were performed on 56 regenerants (sporophytes) and gametophytes maintained in axenic cultures.
The regenerant nuclear DNA content from the TDZ treatments was compared to the respective control plantlets and plantlets derived directly from syngamy and obtained as the result of the long-term culture (additional control). Both controls were maintained on 1/2 MS hormone free medium.
. Results
Shoot Apical Dome Response and Sporophyte Regeneration
Reach spontaneous sporophyte production by clumps of gametophytes appeared to be the only source of sporophytes, which was the supplier of the shoot apical dome (Figure 2A,B). In the control culture on hormone-free medium, the shoot apical meristem (Figure 2C) consisted of a trunk rhizome with leaves and roots. The number of regenerants per explant ranged from one to five. The number of leaves per regenerant ranged from two to seven. The number of roots corresponded to the number of leaves. These regenerants have well-developed filamentous root systems; hence, they are plantlets.
Figure 2
Various stages of the Cyathea smithii culture in the presence of 1/2 Murashige and Skoog (MS) medium supplemented with 2.0% sucrose. (A) Young sporophyte formation (arrows) using a clump of gametophytes during the 6 months of gametophyte initial culture on 1/2 MS medium supplemented with 2.0% sucrose. (B) Numerous sporophytes obtained from single gametophyte clump, which were the source of apical domes. (C) Living specimen of shoot apical dome (star) with leaf primordia (arrows) (Vanox AHBT3; Olympus, Japan). (D) Response of the apical dome on 1/2 MS medium supplemented with 5 µM thidiazuron [TDZ; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea)] and 0.25 µM naphthalene acetic acid (NAA) after 3 months. Note: Developed leaves which determine development were present when explants were isolated and three roots indicated the general organ development pattern. In the center, a huge number of sporophyte primordia is present. (E) Post-TDZ treatment culture of the apical dome maintained on hormone-free medium after 3 months. (F) Response of apical dome on 0.01 µM TDZ and 0.25 µM NAA after 3 months of culture. Note: Development of numerous leaf primordia, indicating numerous young sporophytes with or without roots. (G) Numerous regenerants derived from TDZ culture after 6 months on hormone-free medium. At this stage, the evaluation of developmental data with recognition of particular plants (note their fiddleheads) was done.

At the highest concentration of TDZ (5.0 µM), the primary response of the explant was the overgrowth of the meristem forming a “basket” of primordia with a shortened trunk rhizome and at least three primaries, but not completely developed leaves. In addition, a limited number of thicker roots, usually one or two, with a distinct root meristem zone, maintained the regenerants on the medium. Increasing the level of TDZ increased the average number of regenerated plants by up to 26-fold compared to that of the control. The regeneration potential, expressed by the minimum and maximum regenerated sporophytes, indicated a wide range for different TDZ concentrations. The value of ranges follows the values 1–5 (control), 2–24 (0.01 µM), 31–69 (0.1 µM), 74–95 (1.0 µM), and 64–126 (5.0 µM), respectively, for the control and following from the lowest to the highest concentration of TDZ (Figure 3). At the highest TDZ concentration (5.0 µM), the highest number of sporophytes with at least two to six leaves were produced. The lowest concentration of TDZ (0.01 µM) induced 3–14 leaves per explant, whereas the control culture had 7–21 leaves (Table 1). The highest concentration of TDZ inhibited the growth and development of leaves (Figure 2D,E), whereas the lowest TDZ concentration was similar in leaf size to that of the control culture (Figure 2F). After 6 months of culture, the growth of individual plants in the cluster of regenerants was easily observed (Figure 2G).
Figure 3
The number of the sporophytes regenerated from a single apical dome of Cyathea smithii cultured on hormone free 1/2 Murashige and Skoog agar medium (control – 1) or supplemented with 0.01 (2), 0.1 (3), 1.0 (4), or 5.0 (5) µM thidiazuron [TDZ; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea)], with 0.25 µM naphthalene acetic acid (NAA) (2–5).
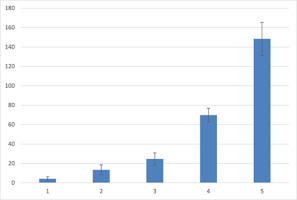
Table 1
The regeneration potential described by the number of regenerants, and the level of their potential expressed by the range of the number of leaves achieved in two steps of culture; first: long-term experiments with the shoot apical dome initially cultured on medium supplemented with various concentration of thidiazuron [TDZ; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea)] + naphthalene acetic acid (NAA) and second: subculture on 1/2 Murashige and Skoog hormone-free medium.
Considering all measured characteristics (Figure 4), it was clear that increasing the TDZ concentration increased the number of regenerants to almost 100 plants per explant. An increased number of regenerants decreased the number of regenerated leaves, indicating a reduction in the developmental potential of the regenerants. Taking into account the length of the regenerated leaves, the highest concentration of TDZ strongly reduced the length of the leaves compared to that in the control and the lowest TDZ concentration (0.01 µM). At the increase of TDZ concentrations, the reduction of the length ranged from 6.4 cm (0.01 µM TDZ) to 2.2 cm (5.0 µM TDZ).
Figure 4
Growth and development of regenerants in post thidiazuron [TDZ; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea)] treatment of the apical dome on 1/2 Murashige and Skoog hormone-free medium considering: (A) number of regenerants per explant, (B) number of leaves per regenerant, and (C) length of the longest leaf (cm) of the regenerant.
Mean ± standard deviation marked by different letters are significantly different (p ≤ 0.05) according to Tukey’s post hoc test.

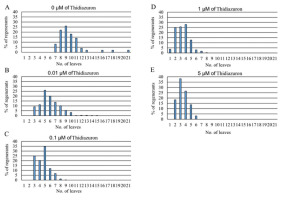
Figure 5 depicts the percentage of regenerants according to their leaf number regeneration potential in medium supplemented with TDZ. In the control, the number of regenerated leaves ranged between seven and 21, with the highest percentage of regenerants having nine leaves. The application of 0.01 µM TDZ decreased leaf formation, ranging from to 3–10 leaves, with the largest percentage of regenerants having five leaves per explant. The application of 0.1 µM concentration decreased leaf formation, ranging from three to eight leaves, with the highest percentage of regenerants having five leaves. The application of 5.0 µM TDZ concentration decreased leaf formation, ranging between two and six leaves, with the highest percentage of regenerants having three leaves. The application of 1.0 µM TDZ decreased leaf formation, ranging between one and seven leaves, and the percentage of regenerants (25% to 28%) with two to four leaves was not significantly different.
Plant Regeneration System and Nuclear DNA Content
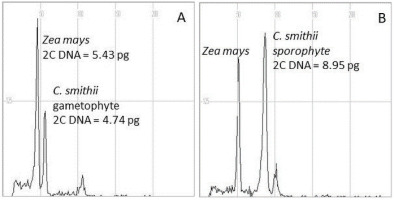
Spontaneous production of C. smithii by gametophytes is the main source of sporophytes. Using 1/2 MS0 medium, gametophytes were maintained for years with at least 6 months of subculture. This protocol did not change the ploidy level of the haploid generation, for which the nuclear DNA content ranged from 4.74 to 4.78 pg (Table 2, Figure 6). In sporophytes, nuclear DNA content ranged from 8.11 to 9.05 pg, with the lowest value for regenerants derived from the culture maintained in the presence of 0.1 µM TDZ.
Table 2
Nuclear DNA content* of Cyathea smithii regenerants derived from different regeneration treatments in the presence of various concentrations of thidiazuron [TDZ; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)-urea)].
* As the internal standard, young leaves of maize (Zea mays L.) CE-777 2C DNA = 5.43 pg (Lysak & Doležel, 1998) were used.
Figure 6
Flow cytometric analysis of nuclear DNA content of regenerants of Cyathea smithii using young leaves of maize (Zea mays L.) CE-777 2C DNA = 5.43 pg (Lysak & Doležel, 1998) as internal standards. (A) Gametophyte and (B) sporophyte.
. Discussion
This study aimed to describe the morphogenic potential thereby experimental value of the tissue complexes of the apical dome meristem of the tree fern C. smithii sporophytes.
Looking at the response (numerous sporophytic regeneration) of C. smithii explants in the control culture (hormone-free medium), the question should be asked whether the object of experiments such as C. smithii was appropriate. The response in the form of a two- to five-fold multiplication of the meristem dome indicates that the species responds very well to two main in vitro treatments: (i) the isolation of the meristem dome and (ii) the requirements for the compatibility of the medium content with the growth requirements of the explant’s meristematic activity.
We were able to use TDZ, which was applied for culture of isolated apical dome (apical initial + subapical initials) (Ambrose & Vasco, 2016) and to create unlimited cell multiplication with the morphogenic potential of fern. In the meristem, the highest TDZ concentration led to the cell multiplication dispersion of the meristem. Increasing the TDZ concentration increased the number of regenerants. 5.0 µM TDZ, the highest concentration tested, appeared to be the best. However, higher TDZ concentrations reduced the development of the regenerants in their earlier stages, which resulted in lower morphologically capable regenerants. 1.0 mg/L TDZ had a significant effect on green globular body induction and multiplication in the tree fern C. barometz (Yu et al., 2017). In the case of both mono- (Malabadi et al., 2011) and dicotyledonous plants (Chhabra et al., 2008) higher TDZ concentrations increased somatic embryogenesis effectivity.
As shown in our previous study, the somatic embryo of C. delgadii (Mikuła et al., 2015) is the result of numerous linear cell divisions initiated by epidermal single cells or cell complexes, which seem to have occurred before tetrahedral cell differentiation. The lack of cytomorphological studies, the fact that only 1/2 MS medium was used to complete the growth and development of regenerants, the potential role of tetrahedral apical meristem cells, and their multiplication has not been solved. We do not know what happens first, the multiplication of the tetrahedral cell followed by multiplication cells of the newly formed meristem or alternatively the cell divisions of the meristematic dome followed by determination of tetrahedral cell differentiation from them.
The growth of regenerants, both in vitro and ex vitro, requires apical and subapical initials to produce leaves and roots, followed by trunk formation. In the current study, we identified apical cells surrounded by active meristematic cells beside them with irregular cell divisions to continue the growth of suspension culture of C. smithii (data not published). In the very early stages of embryogenesis, the tetrahedral cell is formed when the embryo achieves cell determination of organs in the frame of its globular eight-celled stage (Johnson & Renzaglia, 2008). In our experiments, the maximum number of regenerants per apical dome of C. smithii reached 126 individuals (2 in power 6). The numbers indicates that at least six synchronized cell divisions of apical initial would happen to fulfill the need for one tetrahedral cell for one shoot apical meristem, which leads to the growth and development of a regenerant. Concurrently, cells of the subapical initials undergo numerous cell divisions to build numerous cup-shaped zones responsible for leaf primordia differentiation (Ambrose & Vasco, 2016).
In all regenerants, root system formation was based on root regeneration at the bottom of the already-formed leaf. Root system regeneration did not cause any problems, as long as new leaves were regenerated under the conditions of the studied culture system.
Using a reliable system to measure cell DNA uniformity (the nuclear DNA content), we confirmed the diploid level of all sporophyte regenerants, regardless of the TDZ concentration used and the haploid value of all gametophytes analyzed. This method has been adopted from the analysis of red blood cells to studies of the nuclear DNA of plant cells for biosystematics, ecology, population biology (Loureiro et al., 2010), and genetic evaluation of regenerants derived from various ways of plant cell manipulation. Ferns are not plants for which this method is often used; however, the subject presents very interesting fields of studies on the correlation between nuclear DNA content and size of spores (Dyer et al., 2013), analysis of genome size data and chromosome number in a phylogenetic framework of pteridophytes (Chang et al., 2020; Clark et al., 2016), and the relationship between the mean long terminal repeat retrotransposon insertion dates and haploid nuclear genome size (Baniaga & Barker, 2019). In this study, for all 56 analyzed regenerants, the TDZ concentrations used did not affect the nuclear DNA content; however, the growth and development of the regenerants derived from the apical dome were greatly affected by TDZ concentrations. The highest concentration used increased apical meristem multiplication but delayed its development and slowed the growth of regenerants of C. smithii.
. Conclusion
Methods of in vitro culture that increase the morphogenic potential of tree ferns are important for their mass multiplication. While TDZ has rarely been used in biotechnological experiments on ferns, it greatly affects the meristematic activity of apical dome cells and the growth and development of regenerants. Improved plant multiplication will be of value for horticultural businesses propagating ferns, especially as the propagation protocol did not affect the nuclear DNA content of regenerants. In this study, one more species of the tree ferns of the Cyathea genus (C. smithii) was added to a very short list of ferns with known nuclear DNA content.