. Introduction
Botrytis cinerea (Pers.): Fr., the anamorph of Botryotinia fuckeliana(de Bary) Whetzel, is a common and widespread ascomycete, causes gray mold on a wide range of plants including vegetables, fruits, field crops, and ornamental flowering plants (Droby & Lichter, 2007; Jarvis, 1977). It has been ranked second in a list of the world’s top fungal plant pathogens (Dean et al., 2012), as the most important post-harvest pathogen of small fruit crops (Feliziani & Romanazzi, 2016; Gubler et al., 2013; Rivera et al., 2013). In fact, it is also a significant threat to flowering ornamentals such as cyclamen, roses, and gerbera (Pikovskyi et al., 2018). The most common symptoms are soft rot, leaf spots, and necrotic lesions (spots) on different parts of the plant, followed by the rapid appearance of a grey mass of conidia (Williamson et al., 2007).
Oleander (Nerium oleander L.) is also a host plant of Botrytis cinerea. However, the pathogen is rarely a serious problem. In most cases, the symptoms are only observed on flowers and are usually more obvious on light-coloured varieties. Much more frequent damages are caused by Ascochyta oleander (Sacc et Speg), Verticillium dahliae (Kleb), and leaf spot-causing fungal pathogens. In recent years, it has been reported that Botrytis cinerea can infect not only flowers but also woody parts. Symptoms may be emerging on the woody parts (Horváth et al., 2006). However, no significant damage or plant mortality has been noted. In 2020 and 2022, small lesions and brownish necrotic spots were observed on oleander leaves and branches in some plant nurseries in Hungary. Usually, these symptoms are associated with a fungal disease, but the pathogen causing such disease has not been studied in Hungary yet, so the study aimed to identify the pathogen.
. Material and methods
Twenty infected oleander samples, flowering branch shoots, and leaves with small lesions and brownish necrotic spots were collected in hobby gardens in Hungary in 2019–2022. The symptomatic plant parts were delivered to the laboratory of the Department of Plant Pathology, Hungarian University of Agriculture and Life Sciences. The samples were surface disinfected, and the symptomatic parts of the samples were cut out, placed on potato dextrose agar (PDA, BioLab Zrt., Hungary) plates, and incubated at 24 °C. The morphological characterization was determined by the Nikon Eclipse microscope. Cultural characteristics were recorded on PDA plates. To verify the pathogenicity of the isolate mycelial discs from 10 days old, PDA colonies of each isolate were placed on disinfected intact skin tissues of healthy 1-year-old oleander leaves and stems. The control plant organs were treated with sterile PDA discs. The plants were incubated for 14 days at room temperature (25 ± 2 °C).
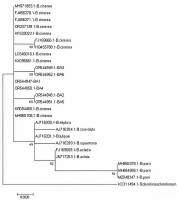
For molecular identification, genomic DNA was extracted from the growing margins of pure cultures using the cetyl-trimethyl-ammonium-bromide (CTAB) method (Maniatis et al., 1983), followed by chloroform/isoamyl alcohol (24:1, v/v) extraction and isopropanol precipitation. Polymerase chain reaction (PCR) was performed to amplify the 18S-28S rRNA region (a partial sequence of the 18S ribosomal gene, the ITS1 region, the 5.8S ribosomal gene, the ITS2 region and partial sequence of the 28S ribosomal gene) using primer pairs ITS5 and NL4 (Tarbell, 2008). The fragments were amplified in a total reaction volume of 40 µl containing 4 µl genomic DNA, 20 µl Dream Taq Master Mix (2X) (Thermo ScientificTM), 2 µl of each primer (20 pmol/µl), and 12 µl sterile water. The PCR conditions included initial denaturation at 94 °C for 5 min, followed by 35 cycles of the following steps: 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 90 s, and a final extension at 72 °C for 10 min. Amplification was verified on a 1% (w/v) agarose gel stained with ECO Safe Nucleic Acid Staining (Biocenter) in 1×TBE buffer. The PCR products were purified using the High Pure PCR Product Purification Kit (Roche Diagnostics GmbH) and cloned into pGEM-T Easy Vector (Pomega). The concentrations of purified PCR products were measured by NanoDrop 2000c spectrophotometer and sent to Base Clear BV in the Netherlands for sequence determination. The obtained sequences were compared to sequences available in the National Center for Biotechnology Information (NCBI GenBank) database, using the Basic Local Alignment Search Tool (BLAST) program. The phylogenetic analysis was carried out using MEGA 7.0 (Molecular Evolutionary Genetics Analysis), using the Maximum Likelihood method and Kimura 2-parameter model (Kumar et al., 2016; Nei & Kumar, 2000). The 18S-28S rRNA region sequences of the newly obtained six Hungarian isolates, twenty Botrytis isolates, and one Slerotinia sclerotiorum isolate available from the GenBank were used for building the tree.
. Results
The symptoms were observed on flowers, leaves, and stems of oleander samples. Small, circular, or elongated brown spots appeared on the petals. Large, watery, brown lesions were formed on leaves and stems. Infected plant parts were often completely covered with a greyish-brown mass of conidia. Infection of the leaves started at the petiole and spread towards the leaf tips. In severe cases, the pathogen invaded the woody tissues, and plants died. The damage was generally greater on young plants.
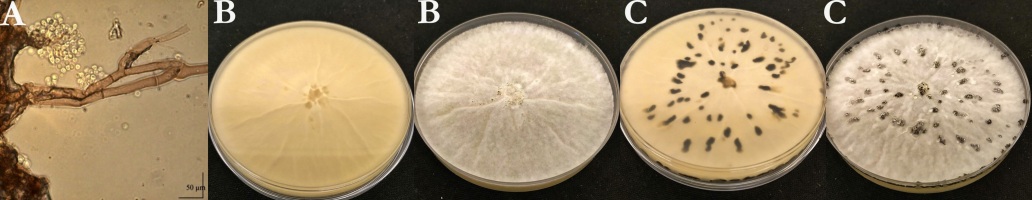
Six isolates (Table 1) were isolated from the infected plant parts on PDA medium. The conidia borne on conidiophores were unicellular, hyaline to pale brown, and elliptical or ovoid with an average measured size of 6.18 × 5.9 µm. The color of the young colony was whitish-grey. Abundant aerial mycelium was on the upper surface. Conidia were observed all over the plates. The pathogen colonized the Petri dishes within 5–7 days. The average daily growth rate of the colonies was 14.63 mm/day. Black-colored sclerotia were formed in the cultures after 3–4 weeks (Figure 1).
Table 1
Data on isolates (Host varieties, collected plant organs, year and place of collection of the isolates).
Figure 1
Morphological characteristics of Ba1 isolate. (A) Conidia and conidiophores; (B) and (C) morphological characteristics of the colony of Ba1 isolate on PDA medium (B: after a week, C: after 4 weeks).
In the pathogenicity tests, the isolates caused brown lesions on the oleander leaves around the infection point 5–6 days after inoculation. A few days later, the size of the necrotic leaf spots increased, and a grey conidia mass appeared on them. The symptoms on oleander stems were similar to those on leaves, observed 10 to 12 days after inoculation. Brown spots were formed at the inoculation points, and later, a grey mass of conidia appeared. After seven days, the stem spots became larger and surrounded the entire stem. They also spread to the healthy leaves. Control leaves and stems did not show any symptoms (Figure 2). Koch’s postulates were fulfilled by the re-isolation of Botrytis cinerea from the symptomatic tissues of the inoculated leaves and shoots. The re-isolated pathogens had the same morphological characterization as the fungi used for the pathogenicity testing.
Figure 2
Pathogenicity test on oleander. (A) Control inoculations; (B) Oleander leaf inoculated with Ba3 isolate, six days after inoculation; (C) Oleander leaf inoculated with Ba3 isolate, 14 days after inoculation; (D) Oleander shoot inoculated with Ba6 isolate, 10 days after inoculation; (E) Oleander shoot inoculated with Ba6 isolate, 20 days after inoculation.

The obtained sequences were ∼1,200 bp in length. All the sequences were uploaded to the NCBI GenBank database (Acc. number: OR544947, OR544948, OR544949, OR544950, OR544951, OR544952). The Hungarian isolates were located on a separate branch of the tree with the other Botrytis cinerea isolates. The nucleotide sequence identities were 99–100% between the Hungarian isolates and the other Botrytis cinerea isolates from different host plants. Nucleotide sequences of Ba4-Ba1, Ba5-Ba2, and Ba3-Ba6 isolates were shown to be 100% identical and distinct from the other Botrytis species (Figure 3).
. Discussion
Although Botrytis cinerea is an important pathogen of numerous crop species (such as vegetables, ornamentals, field, storage, and transport crops) worldwide (Elad et al., 2004), no morphological or molecular study has been carried out on isolates from oleander in Hungary. Based on the morphological characteristics, the fungi isolated from oleander can be identified as Botrytis cinerea. The conidial dimension and the cultural features of the pathogens were consistent with the literature data (Glits & Folk, 2000; Husz, 1952; Jarvis, 1977; Mirzaei et al., 2008). However, these morphological characteristics could be considerably modified by environmental and cultural conditions. Menzinger (1966) found that the dimension and shape of the conidia, and the characters of mycelia and sclerotia could be influenced by cultural conditions. Vanev (1972) also reported how the temperature and culture medium could change the conidial size, form, and colony characters. Therefore, in addition to examining morphological characters, molecular studies are also required for proper identification of Botrytis species.
The pathogenicity of the isolates was confirmed on oleander. Brown necroses were observed on the leaves, and brown spots on the branches around the inoculation points. A grey mass of conidia appeared on the parts of the infected plant. The control plant showed no symptoms. The Koch’s postulates were fulfilled. The nucleotide sequences of the ITS1 regions were 99–100% identical to the sequences of several isolates of Botrytis cinerea. They were located on a separate branch from the other Botrytis species, and Sclerotinia sclerotiorum isolate. Based on the morphological and molecular characteristics of the pathogens, all six isolates were identified as Botrytis cinerea (Pres).
Botrytis disease of oleander was first reported in a study of fungal diseases in Sierra Leone in 1936 (Deighton, 1936). Since then, only two publications have mentioned the pathogen on Nerium oleander (Dudka et al., 2004; French, 1989). In the Hungarian literature, the symptoms of oleander were mentioned by Horváth et al. (2006). However, significant damage with necrotic spots on branches, which can be the cause of plant mortality, has not been reported yet. To the best of our knowledge, this is the first study in our country aimed at the identification of Botrytis cinerea isolated from oleander by classical and molecular mycological methods.