Introduction
Hypogeous macrofungi are rarely considered in mycological investigations. Although considerable progress in knowledge of their taxonomy, diversity, and distribution has been made in recent years (e.g., Alvarado et al., 2016; Moreau et al., 2011; Paz et al., 2016, 2017; Stielow et al., 2011; Vidal et al., 2019), they remain one of the most understudied groups of fungi in Poland and other Central European countries.
Over 20 species of hypogeous Basidiomycota and nearly 30 species of hypogeous Ascomycota were found in Poland before the year 2000 (Chmiel, 2006; Ławrynowicz, 1988; Wojewoda, 2003). Since then, several studies have reported on the localities of new species hitherto not known to occur in Poland (e.g., Chachuła, 2018; Chachuła et al., 2020; Hilszczańska et al., 2008; Mleczko et al., 2009, 2010; Paz et al., 2017; Vidal et al., 2019; Wojewoda et al., 2016). Some recorded fungi are widespread and, at least locally, common; for example, Elaphomyces asperulus, E. granulatus, and Octaviania mutabilis (as O. asterosperma) (Ławrynowicz, 1988; Mleczko et al., 2009). The status of certain species regarded as very rare changed when more data on their distribution became available; for example, Chamonixia caespitosa (Kozak et al, 2013; Mleczko et al., 2010). However, several species have only been found in a single or only a few localities in Poland, for example, Elaphomyces aculeatus, Gautieria othii, Lactarius stephensii, Russula candidissima, and R. cerea (Chachuła et al., 2020; Ślusarczyk, 2018; Vidal et al., 2019).
The aim of this study was to report on new localities of three rarely recorded species in Central Europe: Leucangium carthusianum, Melanogaster luteus, and Rhizopogon melanogastroides. Two of which, M. luteus and R. melanogastroides, are reported here for the first time in Poland.
Methods
Sporocarps of hypogeous fungi were found during field work carried out in southern Poland, in the Polish Carpathians in particular, in the years 2011–2019. The sporocarps were excavated by the careful removal of litter and outer soil layers to a depth of a few centimeters with the aid of rakes from an area of approximately 0.5 m2. After exploration, the soil and litter were replaced in the excavation area.
The geographical coordinates of the localities were established using GPS Garmin 62s. Information regarding the type of plant community, associated ectomycorrhizal plant species, and elevation were noted. When possible, photos of the sporocarps were taken with a digital Nikon D90 camera with a Sigma 17–70 mm lens.
The identification of sporocarps was based on contemporary keys and monographic papers of Martín (1996), Montecchi and Sarasini (2000), and Moreau et al. (2011). Macroscopic features were observed on fresh and dry specimens. Microscopic features were observed using a ZEISS AxioScope A1 light microscope with DIC and digital camera (AxioCam MRc5) on handmade preparations of water-rehydrated sporocarps and were mounted in water, 3% KOH, and Melzer’s reagent. The measurements of the microscopic structures necessary for sporocarp identification were performed using the computer image acquisition and analysis software ZEN 2012, blue edition (Carl Zeiss Microscopy). Collections of identified sporocarps are preserved in the Herbarium of the Institute of Botany, Jagiellonian University in Kraków (KRA), Poland.
Information regarding the locality, presented below, includes: (i) the square in the ATMOS grid (Wojewoda, 2000), (ii) the macro- and mesoregion, (iii) data of the locality (the name of the village, nature reserve, hill, mountain, valley, etc.), (iv) plant community type or plant association (if known), (v) associated ectomycorrhizal tree species, (vi) elevation, (vii) collection date, (viii) names of collecting and identifying persons, and (ix) herbarium specimen number. Where a locality is reported in a previous study, the source of information is quoted.
The notes present information on characteristic features, taxonomic position, ecology, and known distribution of species in Central Europe in particular. Information regarding synonyms is according to the Index Fungorum and MycoBank, unless specified otherwise.
Species and Localities
Leucangium carthusianum (Tul. & Tul.) Paol., Syll. fung. 8: 900 (1889)
Synonyms: Picoa carthusiana Tul. & Tul., Fungi hypog, ed. alt.: XXIV (1862), L. ophthalmosporum Quél., C. r. Assoc. Franç. Avancem. Sci. 11: 404 (1883) [1882]
Known Localities in Poland (Figure 1A)
Fd-38 KRAKÓW-CZĘSTOCHOWA UPLAND, Olkusz Upland: Trzyciąż planned reserve, in mixed forest (Abies, Picea, Acer pseudoplatanus), 400 m a.s.l., September 2013 (Chachuła, 2018).
New Localities (Figure 1A)
Bf-47 EASTERN SUDETES, Śnieżnik Mt: Kletno, in mixed forest (Picea, Fagus), 710 m a.s.l., 2013-07-20, leg. & det. M. Kozak & K. Kozłowska-Kozak, KRA F-2013-35.
Gd-17 OUTER WESTERN CARPATHIANS, Beskid Żywiecki Mts, Babia Góra Range, Skawica, in the middle part of the Skawiczanka Stream Valley, in mixed forest (Abies, Fagus, Picea), 550 m a.s.l., 2012-06-28, leg. & det. M. Kozak, KRA F-2012-38.
Gd-59 CENTRAL WESTERN CARPATHIANS, Western Tatra Mts: Tatra National Park:
Wielka Sucha Dolina Valley, in coniferous forest (Abies, Picea), on calcareous bedrock, 970 m a.s.l., 2019-09-24, leg. & det. M. Kozak & F. Karpowicz, KRA F-TPN/19/0357;
N slope of Hruby Regiel, above road from Zakopane, in coniferous forest (Abies, Picea), under Abies, 980 m a.s.l., 2019-10-15, leg. & det. M. Kozak & J. Brańka, KRA F-TPN/19/0471.
Ge-11 OUTER WESTERN CARPATHIANS, Gorce Mts: mouth of Za Palacem Stream, in garden near buildings, close to the forest edge (Abies, Picea, Fagus), 650 m a.s.l., 2015-11-09, leg. E. Zając, det. P. Mleczko, KRA F-2015-3.
Ge-21 OUTER WESTERN CARPATHIANS, Gorce Mts:
Gorce National Park, upper part of Duże Jaszcze Stream Valley, in Carpathian beech forest (Dentario glandulosae-Fagetum association), 790 m a.s.l., 2015-08-17, leg. & det. M. Kozak, KRA F-2015-36;
Left slope in the middle part of the Forędówki Stream Valley, in mixed forest (Abies, Picea, Fagus), on steep slope by the small stream, 860 m a.s.l., 2012-11-10, leg. & det. M. Kozak & K. Kozłowska-Kozak, KRA F-2012-112.
Notes
The features of the Polish specimens correspond to those published in other studies, for example, Montecchi and Sarasini (2000) and Van Vooren (2017). Tuber-like sporocarps, which can reach over 5 cm in diameter, vinaceous-black-to-black peridium with a granular-to-warty surface, and cream-colored, solid gleba that turns gray with age in fertile chambers, are macroscopic characters of the species (Figure 2A). However, the most distinguishing features are lemon-shaped, deeply yellow-to-yellow-brown, guttulate spores with dimensions of approximately 65–70 × 20–35 μm (Figure 2E,F).
Originally described as a new species of Picoa by Tulasne & Tulasne (1862), the species was then classified within the genus Leucangium by Paoletti (Saccardo, 1889). Recent phylogenetic studies based on multilocus sequence analysis have confirmed its position within the Morchellaceae-Discinaceae lineage in the Morchellaceae family, whereas species of Picoa, such as P. lefebvrei and P. juniperina, have been placed in Pyronemataceae (O’Donnel et al., 1997; Sbissi et al., 2010; Trappe et al., 2010).
The first record of this species in Poland comes from the Olkusz Upland, a calcareous region in the central part of the country (Chachuła, 2018). Several other localities were discovered in southern Poland, mostly in the Carpathians but also in the Sudetes (see the list above). The species is associated in Europe with the coniferous trees A. alba and P. abies (e.g., Montecchi & Sarasini, 2000). However, in North America, it is a mycorrhizal partner of Pseudotsuga menziesii (Palfner & Agerer, 1998; Trappe, 1971; Trappe et al., 2010). In North Africa, L. carthusianum grows near Rhanterium suaveolens and Helianthemum lipii in calcareous alkaline soils, but it could still be a separate taxon (Thomas et al., 2019).
The species is widespread but rare in Europe (Montecchi & Sarasini, 2000; Van Vooren, 2017). Most reports regarding its taxonomy and distribution are provided by MycoDB [“Base de Jacques Trimbach (Picoa carthusiana),”2020], Oertel (2011), and Van Vooren (2017). Other than Southern and Western European countries, such as France (Van Vooren, 2017), Italy (Montecchi & Sarasini, 2000), Spain, and Switzerland (Flammer, 2011; Senn-Irlet, 1985), it has also been found in all Central European countries, including Austria (“5976. Picoa carthusiana Tul.,” 2020), the Czech Republic (Valda, 2018; Veselý, 1928), Germany (“Picoa carthusiana Tul. & C. Tul. 1862,” 2020), Hungary (Bratek et al., 2013), Slovakia (Glejdura, 2013; nahuby.sk, 2020; Pejger, 2019), northern Romania (Béres, 1999), and Poland. Most localities are present in mountain ranges, for example, the Alps in southern Germany and Austria, the Sudetes in southeastern Germany and Poland, and the Carpathians in Hungary, Romania, Slovakia, and southern Poland. However, it has also been found in low elevation areas, such as the Moravia and Prague regions in the Czech Republic and the Olkusz upland in Poland. It has been recorded in both calcareous regions, such as the Olkusz upland, Tatras, and Alps, and on acid, volcanic bedrocks, for example, the Stolicke vrchy in Slovakia (Glejdura, 2013) and the Sudetes.
Rhizopogon melanogastroides M. Lange, Beihefte zur Sydowia 1: 255 (1957)
Synonyms: -
New Localities (Figure 1B)
Gd-59 CENTRAL WESTERN CARPATHIANS, Western Tatra Mts: Tatra National Park: left slope of Strążyska Valley, on a limestone rock, under Pinus mugo, 1,130 m a.s.l., 2019-10-14, leg. M. Kozak & J. Brańka, det. P. Mleczko, KRA F-TPN/19/0056.
Notes
The species was described by Lange in 1957, who based its description on the specimen collected by Franz Petrak in Moravia. Because of the peculiar bullet-shaped thick-walled pigmented spores, it was believed to occupy a transitional position between the genera Rhizopogon and Melanogaster (Lange, 1957; Martín, 1996; Trappe, 1975). In the monograph of the European species of Rhizopogon, Martín (1996) included R. melanogastroides in this genus; however, phylogenetic analysis based on the ITS nrDNA region suggested that the taxon should be placed outside Rhizopogon (Martín & Raidl, 2002). The results of molecular studies as well as the features of the ectomycorrhizas were arguments against including this species in the genus Melanogaster. Indeed, although peculiar due to the presence of globular cells in the outer mantle, the ectomycorrhizas of R. melanogastroides share some important features with other Rhizopogon species, that is, a lack of sclerotia, clamps, and globular to capitate cystidia (typical for Melanogaster ectomycorrhizas) and the presence of a gelatinous matrix in the outer mantle (Agerer, 2006). In the phylogenetic analyses based on the LSU nrDNA region, performed by Binder and Hibbett (2006), it was found to be nested within the genus Rhizopogon.
This species is extremely rare in Europe. Long known only from the historical type locality in Moravia, in the municipality of Hranice (German: Mährisch Weißkirchen), and in the Moravian Gate between the Carpathians and the Sudetes (Lange, 1957; Martín, 2001), it was then discovered in 1997 in the Bad Tölz-Wolfratshausen District, Bavaria, Germany, in the Northern Limestone Alps (Martín & Raidl, 2002; Raidl et al., 1998) and 10 years later, in 2007, in Cancano, the province of Sondrino, Lombardy, Italy, in the Southern Limestone Alps (Bincoletto, 2014). In all three localities, sporocarps were found in limestone areas under Pinus: in the Czech Republic – in open slopes, under undetermined Pinus species, in a mixed wood dominated by Quercus sp. (Lange, 1957); in Germany, in a loose stand of Picea abies, Pinus sylvestris, P. mugo, and some shrubs (Erico-Pinetum plant association), close to the bank of the river Isar, at 795 m a.s.l. (Martín & Raidl, 2002); in Italy, in an alpine grassland with Dryas octopetala, Salix serpyllifolia, Gentianella ciliata, G. campestris, Leontopodium alpinum, and Chamorchis alpina, at ca. 2,000 m a.s.l. (Bincoletto, 2014). The Polish locality is also placed in an exposed limestone rock with scattered P. mugho at 1,130 m a.s.l. in the mountain valley.
Lange based his description of the new species on exsiccatum, which consisted of one well-preserved sporocarp, deposited in the Botanical Museum of the University of Copenhagen (Museum Botanicum Hauniense). Macromorphological characters were then completed by the description of fresh sporocarps from Germany and Italy (Bincoletto, 2014; Martín & Raidl, 2002; see Table 1). The study of Italian sporocarps revealed a strong dextrinoid reaction of young, hyaline spores that cannot be observed in mature, pigmented ones. Analysis of sporocarps from the Tatra Mts confirms this statement (Table 1, Figure 3D). We also found no bluing reaction of mature sporocarps after touching, reported for the material collected in Germany (Martín & Raidl, 2002), However, we found a reddish peridium reaction after the application of 10% KOH, as reported by Bincoletto (2014), which can also be tested in dry specimens. Macro- and microcharacters of specimens from Tatra Mts are generally in agreement with those presented in previous studies (Table 1, Figure 2C,D, Figure 3C–H).
Melanogaster luteus Zeller, Mycologia 31(1): 9 (1939)
Synonyms: Alpova luteus Trappe, Beihefte zur Nova Hedwigia 51: 291 (1975), Melanogaster microsporus Mattir., Beiträge zur Kryptogamenflora der Schweiz 8(2): 39 (1935), Alpova diplophloeus f. europeus Trappe, Beihefte zur Nova Hedwigia 51: 289 (1975)
New Localities (Figure 1C)
Ge-50 CENTRAL WESTERN CARPATHIANS, Western Tatra Mts: Tatra National Park: S slope of Kopieniec Wielki Mt, artificial stand (Alnus incana, Larix sp.) in a calcareous sward representing Seslerion tatrae alliance, 1,300 m a.s.l., 2019-08-26, leg. F. Karpowicz & K. Kozłowska-Kozak, det. P. Mleczko, Filip Karpowicz, KRA F-TPN/19/0006.
Ge-51 CENTRAL WESTERN CARPATHIANS, High Tatra Mts: Tatra National Park: mouth of Waksmundzki Stream, Alnetum incanae association (Alnus incana, Picea), 1,000 m a.s.l., 2019-09-21 (found in two localities), leg. & det. M. Kozak, KRA F-TPN/19/0291, KRA F-TPN/19/0292.
Gf-46 OUTER EASTERN CARPATHIANS, Western Bieszczady Mts: Łubne, mouth of the Kotanica Stream, in Alnetum incanae association (Alnus incana, Tilia, Corylus), 520 m a.s.l., 2012-10-22, leg. M. Kozak & K. Kozłowska-Kozak, det. M. Kozak & P. Mleczko, KRA F-2012-86.
Notes
The taxonomy of the Alnus-associated species of Boletales related to Paxillus, that is, Alpova and Melanogaster, has long been very complicated and unclear due to uncertain descriptions and misleading interpretations of names and species concepts (Moreau et al., 2011). For that reason, reconstruction of the distribution of species in this group is difficult and often requires the study of original collections. This is also true for M. luteus, which is often referred to as Alpova diplophloeus, especially if specimens are identified according to Kers (1986), Knudsen (2012), Montecchi and Lazzari (1989), and Montecchi and Sarasini (2000). Another name used for this taxon in the European keys is M. microsporus (e.g., Eckblad & Lange, 1997).
The features of the Carpathian specimens correspond well with the description published by Moreau et al. (2011). Small, round-to-slightly flattened sporocarps of up to 1.5 cm in diameter without columella, greenish-yellow-to-greenish-brown, a furfuraceous-to-stringy peridium surface, black, deliquescent, mature gleba with whitish-to-pale yellow septa are characteristic macromorphological features of this species (Figure 2B). In the micromorphological aspect, the most distinguishing features are: peridium that is plectenchymatous throughout (pseudoparenchymatous in the inner layer in Alpova) (Figure 3A,B), yellow-brown, elongated spores 5.3–6.8 × 2.4–3.3 μm with remnants of hyaline sterigmata at their bases (Figure 2G,H), basidia with thin walls and lack of spherical “supporting cells” in the gleba (characteristic for Alpova spp.). The closely related species M. rivularis, known to date only from Corsica, differs in bigger sporocarps, over 1.5 cm in diameter, with distinct columella and basidia with thick walls at their apices (Moreau et al., 2011).
The presence of M. luteus was confirmed in France, Italy, Montenegro, and Scandinavia; however, it is most likely to be more frequent but rarely searched for (Moreau et al., 2011). The only confirmed localities in Central Europe are those in Slovakia (nahuby.sk, 2020), and now its presence has been confirmed in Poland in the Central Western and Outer Eastern Carpathians. The Polish locality in the Bieszczady Mts is the most extreme eastern location known so far. The most frequent mycorrhizal partner of M. luteus is Alnus incana in alluvial places in rivers or stream banks in mountainous areas (Moreau et al., 2011).
Figure 1
Distribution maps of three hypogeous fungi in Poland: (A) Leucangium carthusianum; (B) Rhizopogon melanogastroides; (C) Melanogaster luteus. The points correspond to the ATMOS squares with localities (see the lists of localities). White dots – new localities; black dot – known locality.
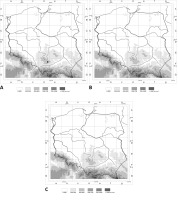
Figure 2
Sporocarps and spores of hypogeous fungi: (A) Sporocarp of Leucangium carthusianum; (B) sporocarps of Melanogaster luteus; (C,D) mature (C) and immature (D) sporocarps of Rhizopogon melanogastroides; (E) spores of L. carthusianum; left – spore from fresh specimen, right – spore from dry specimen (8-years old); (F) ascus of L. carthusianum; (G,H) spores of M. luteus. Scale: (A–D) 10 mm; (E–H) 10 μm. Photo: (A–D) M. K.; (E–H) P. M. (A) KRA F-TPN/19-0357; (B,G,H) KRA F-TPN/19-0006; (C,D) KRA F-TPN/19-0056; (E,F) KRA F-2012-38.

Figure 3
Micromorphological structures of hypogeous fungi: (A,B) Cross section of outer (A) and middle (B) peridium layers of Melanogaster luteus; (C,D) mature (C) and immature (D) spores of Rhizopogon melanogastroides (left – in water, right – in Melzer’s reagent); (E) basidia of R. melanogastroides (first from the left – immature basidium); (F) section of dissepiment of R. melanogastroides; (G,H) section ofthe inner (G) and outer (H) peridium layers of R. melanogastroides. Scale: 10 μm. Photo: P. M. (A,B) KRA F-TPN/19-0006; (C–H) KRA F-TPN/19-0056.
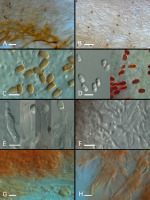
Table 1
A synoptic table of macro- and microcharacters and chemical reactions of Rhizopogon melanogastroides according to descriptions of specimens from the Czech Republic (Lange, 1957), Germany (Martín, 1996; Martín & Raidl, 2002), Italy (Bincoletto, 2014), and Poland (the present study).
Discussion and Conclusions
Investigations carried out in recent years have identified new localities of some hypogeous fungi, which are new for the mycobiota or have not been recorded for over 50 years in Poland. Most species, e.g., E. aculeatus, Gautieria trabutii, Hydnotrya bailii, Hymenogaster luteus, Hysterangium nephriticum, Lactarius stephensii, Melanogaster tuberiformis, Octaviania lutea, Pachyphlodes conglomerata, P. citrina, Russula candidissima, R. mattirolana, Sclerogaster hysterangioides, Tuber bellonae, T. excavatum, T. ferrugineum, T. fulgens, and T. macrosporum, have been found either in the Carpathians or in the upland regions in the middle and middle-eastern part of Poland (the Śląsko-Krakowska Upland, Małopolska Upland, Lubelsko-Lwowska Upland, and Polesie region) (Chachuła et al., 2020; Gierczyk et al., 2019; Hilszczańska et al., 2008, 2013, 2014; Kujawa & Gierczyk, 2011; Ławrynowicz, 2009; Ławrynowicz et al., 2008; Rutkowski et al., 2014; Vidal et al., 2019; Wojewoda et al., 2016). Reports on single species have also come from other regions, such as the Eastern Sudetes (R. cerea), Lubuskie Lake District (Gautieria otthii), or Gdańsk and Wschodniopomorskie Lake District (Melanogaster macrosporus) (Kujawa & Gierczyk, 2011, 2013; Ślusarczyk, 2018; Vidal et al., 2019). Such geographical patterns reflect the activity of researchers; however, data regarding the distribution of hypogeous fungi in Poland seem to indicate that the regions mentioned above, that is, the Carpathian Mts and the calcareous uplands in the central and southeastern parts of the country, are hot spots of diversity for this fungal group in Poland.
In this study, we present new localities of three species, two of which are M. luteus and R. melanogastroides, which are new for Poland. Both were recorded in the Carpathian Mts and both appear to have specific and rather narrow ecological requirements. In the most recent records in Europe, including the Polish record, R. melanogastroides was found only in the mountain habitats on calcareous bedrock, in association with the dwarf pine P. mugho. The occurrence of M. luteus in Europe is restricted by its association with its mycorrhizal partner, the genus Alnus, specifically A. incana, a species characteristic of stream bank habitats in mountain areas. Indeed, Polish localities of M. luteus fit well with this distribution pattern. In contrast to the above-mentioned species, the sporocarps of L. carthusianum were found in mixed and coniferous forests not only in mountain regions but also in the Kraków-Częstochowa Upland. The species seems to be associated primarily with Abies alba, a tree found in almost all known localities of L. carthusianum; however, Picea is the second possible mycorrhizal partner of this species, as it is the only coniferous tree found in the locality in the Sudetes.
The data presented in this article broaden our knowledge regarding the diversity and distribution of hypogeous fungi in Poland. Together with recent findings, our records indicate that the entire hypogeous fungal diversity of the Polish mountains and uplands has not yet been discovered, and new findings can be expected in future.
Handling Editor
Andrzej Szczepkowski; Warsaw University of Life Sciences – SGGW, Poland; https://orcid.org/0000-0002-9778-9567
Authors’ Contributions
PM: study concept, field research, species identification, preparation of manuscript, figures and table; MK: study concept, field research, species identification, preparation of distribution maps; FK: study concept, field research, editing and correcting of the final version of the manuscript


