. Introduction
Rice (Oryza sativa L.) is a major cereal crop that serves as a staple food for 60% of the world population (Nahar et al., 2016). Worldwide rice production in 2020 was as high as 510.6 million tons, which is an increase of 1.8% compared to that in 2019 and an all-time high. This is mainly due to increased yields in China (mainland), the Philippines, Guinea, the Democratic Republic of Congo, and the Bolivarian Republic of Venezuela (FAO, 2021).
In Morocco, the total area of rice crop reached 7,973 hectares with a production of 64,598 tons (FAOSTAT, 2020). Nevertheless, rice domestic consumption is considered to be one of the lowest in the world (1.2 kg of rice per capita), which represents a major constraint to rice production in the country (Food and Agriculture Organization of the United Nations, 2003).
Rice crop is facing several threats, such as diseases caused by fungi, bacteria, and viruses, which affect grain production and quality (Ou, 1985). Helminthosporium disease, caused by Bipolaris oryzae (Breda de Haan) Shoemacker, has been reported in all rice-producing countries (Ou, 1985). The disease is responsible for heavy losses in grain yield (up to 90%) (Sunder et al., 2014). In Morocco, it was first reported in 1996 by Bouslim (1996).
Many studies have reported the presence of B. oryzae in rice. It was detected on rice leaves, stems, and grains (Bahous et al., 2003; Benkirane, 1995; Benkirane et al., 1998, 2000; Gnancadji-Andre et al., 2005; Serghat, Mradmi, et al., 2005; Tajani et al., 2001). The disease symptoms appear on the coleoptiles, leaf sheath, and blade as brown spots with gray or whitish centers, cylindrical or oval in shape (Chauhan et al., 2017).
The optimum temperature for B. oryzae growth and its conidial germination ranges from 27 to 30 °C and 25 to 30 °C, respectively (Bouslim et al., 1997; Lucas et al., 1992; Ou, 1972, 1985). Infection requires a relative humidity of above 89% at 25 °C (Angladette, 1966; Ou, 1985). The conidia of B. oryzae can remain viable for 4 years on infected kernels as well as on healthy-looking kernels (Ou, 1985). This parasite is also conserved in soil (Lucas et al., 1992) and on crop remains (Nyvall et al., 1995).
According to Lage (1997), the presence of Pyricularia infection, Helminthosporium disease, and weeds (Echinochloa crus-galli, Panicums spp., Typha spp., and Cyperus spp.) could slow down rice production (Boulet & Bouhache, 1990). The Food and Agriculture Organization of the United Nations (FAO) stated in 2003 that the most common weed species affecting rice in the Mediterranean region belong to Poaceae and Cyperaceae. In the Gharb region, the most common weeds are Panicum (P. repens, Ligustrum obtusifolium Del.), Typha (T. latifolia L., T. marsii Bat.), Scirpus spp., Cyperus spp., and Echinochloa spp. (Miège, 1951). These species are well adapted to the different agroecosystems where rice is cultivated and can promote the conservation and multiplication of pathogenic species (Pugh & Mulder, 1971; Singh et al., 2008).
Benkirane et al. (2000) observed that Moroccan isolates of Pyricularia oryzae, originating from Stenotaphrum secundatum, are pathogenic on rice. Likewise, Serghat, Mradmi, et al. (2005) found that the fungal pathogen Pyricularia oryzae, isolated from Echinochloa phyllopogon and Phragmites australis, induced leaf lesions and sporulate on the foliage of certain rice varieties.
The leaves of Typha latifolia, a perennial plant species found around rice fields, often show similar leaf lesions to those observed in rice plants infected with B. oryzae. In this study, B. oryzae isolates obtained from leaf lesions in T. latifolia were subjected to pathogenicity tests on the leaves of five rice varieties. Indeed, the objective of this study was to highlight the role of an infectious reservoir, such as T. latifolia, in harboring and spreading leaf pathogens, particularly B. oryzae, to neighboring rice fields, which will cause significant damage to the rice fields.
. Material and Methods
Sampling
The study was carried out during 2014–2016 in the Moroccan North-West (Gharb), including the Souk Tlet region (latitude 34°36′6.153″ N, longitude 6°10′27.687″ W) and the Merjas of Kenitra (latitude 34°30′54.838″ N, longitude 5°52′59.243″ W). Typha latifolia samples were randomly taken from different plots using the diagonal sampling technique (five samples per plot). White plastic bags (70 cm × 50 cm) containing the samples were transferred to the laboratory for analysis.
Mycological Analysis
Infected T. latifolia plants showing leaf symptoms were transferred to the laboratory for microscopic examination, isolation, purification, and pathogenicity test. The blotter method was used for the isolation of B. oryzae. Infected leaf tissues of T. latifolia were collected, cut into small pieces, sterilized by immersion in 0.5% sodium hypochlorite for 1–5 min, and then washed three times with sterile distilled water. The leaf fragments (1 cm in diameter) were placed in sterile Petri dishes containing two discs of filter paper moistened with sterile distilled water. The dishes were then incubated at 22 °C in alternating 12-hr light and darkness. After 48 hr, the leaf fragments were examined under an optical microscope at magnification ×100 for observation of the presence of fungal spores. The fungal species was determined using identification keys (Ou, 1985; Tarr, 1962).
Bipolaris spores were then single-spored with a capillary tube, placed on agar medium (agar-agar: 15 g, distilled water: 1,000 mL), and subsequently transferred using a sterilized needle to the surface of a rice flour-based medium (rice flour: 14 g, agar-agar: 15 g, yeast extract: 4 g, distilled water: 1,000 mL). Four isolates of B. oryzae were cultured.
Pathogenicity Test
The seeds of five rice varieties, namely, Elio, Taibonet, Arpa, Eurano, and Cererrer, were disinfected by immersion in 5% hypochlorite sodium solution for 2 min, followed by three rinses with sterile distilled water. They were then dried on a sterile filter paper and pre-germinated on Petri dishes 90 mm in diameter containing sterile cotton soaked with sterile distilled water. After 72 hr of incubation in the dark at 28 °C, the obtained plantlets were transplanted into pots filled with Mamora soil and then placed in a greenhouse. The seedlings were watered with tap water until they reached the stage required for inoculation, that is, when they had grown four to five true leaves.
Inoculum Preparation
Inoculum was prepared by independently growing each of the four isolates of B. oryzae (Hor1, Hor2, Hor3, Hor4) on a rice flour-based medium (rice flour: 15 g; Agar-agar: 15 g; yeast extract: 4 g and 1,000 mL of distilled water), which is favorable for the sporulation of B. oryzae, for 15 days (continuous photoperiod, 25 °C). After incubation, the cultures were flooded with 15 mL of distilled water, and spores were dislodged using a sterile spreader.
Afterward, the fungal suspension was filtered through a fine mesh cloth to separate the spores from the mycelial fragments. The concentration of the conidia was adjusted to 105 conidia/mL using Malassez slide by adding sterile distilled water supplemented with a drop of Tween 20 and 0.5% gelatin.
Inoculation of Typha latifolia With Spore Suspension
Healthy leaves were soaked in the conidial suspension of each B. oryzae isolate. Control leaves were soaked in distilled water containing a drop of Tween 20 and gelatin. The leaves were placed in 9-cm-diameter Petri dishes containing glass beads moistened with sterile distilled water and then incubated under continuous white light at room temperature for 7 days.
Inoculation of Typha latifolia With Mycelial Discs
Healthy leaves were placed in 9-cm-diameter Petri dishes containing glass beads in the presence of sterile distilled water. They were then inoculated with the mycelial plug (5 mm in diameter) of each isolate: one mycelial plug was placed on the central part of leaf segment, and another mycelial plug was deposited near the leaf apex. Noninoculated leaves (treated with water agar discs only) served as a control. In both detached leaf assays, controls were treated with sterile distilled water containing 0.01% Tween 20. Inoculated and control leaves were kept at ambient temperature under a natural light/dark cycle in the laboratory.
Inoculation of Rice Varieties
Rice seedlings at the stage of five to six leaves were inoculated by foliar spraying of 60 mL of spore suspension at 105 conidia/mL concentration using a hand compressed spray. Control plants were sprayed with sterile distilled water containing Tween 20 and gelatin. After spraying, all plants were covered with a black plastic bag and placed in a greenhouse for the development of symptoms. The plastic bag was used for the first 48 hr to ensure 100% relative humidity during conidium germination and fungal penetration. The replication consisted of three pots with three plants per pot for each rice variety. The experiment was repeated three times.
Assessment of Infection Severity
The degree of leaf necrosis was evaluated on the seventh day after inoculation for the four rice varieties artificially inoculated with conidial suspension, and at 2 days later for the rice inoculated with mycelial plugs. Disease severity was assessed as the proportion of infected leaf area on randomly selected rice plants. It was estimated using a disease rating scale of 1–9, as suggested by Notteghem et al. (1980), on the last two leaves of each infected rice plant. The results are described in Table 1.
Table 1
Disease rating scale (Notteghem et al., 1980).
| Note | Diseased leaf area (%) |
|---|---|
| 0 | 0.00 |
| 1 | 0.05 |
| 2 | 0.50 |
| 3 | 1.50 |
| 4 | 3.50 |
| 5 | 7.50 |
| 6 | 17.50 |
| 7 | 37.50 |
| 8 | 62.50 |
| 9 | 87.50 |
For analysis, the severity scale was converted into Percentage Severity Index (IS) using the following formula:
where xi: disease severity scale; ni: number of infected plants (or leaves) with a rating of i; Nt: total number of plants observed; 9: maximum disease severity scale.
Sporulation on the Host Plant
Sporulation was determined according to the technique of Hill and Nelson (1983) by estimating the average number of conidia produced per unit area of the infected leaves (expressed in number of spores/cm2).
At 7 days after inoculation, rice leaves that showed lesions were removed, cut into four–five fragments, and then placed in Petri dishes containing filter paper moistened with sterile distilled water (one sheet per dish). The dishes were placed under continuous fluorescent light for 72 hr at 28 °C.
Each leaf segment was then collected in a test tube containing 1 mL of sterile distilled water. After that, the tubes were agitated in a vortex mixer for 2 min to detach the conidia from the mycelium. The conidia of the pathogen were counted using a Malassez slide under an optical microscope, with 10 counting for each sample.
. Results
Morphological Characteristics
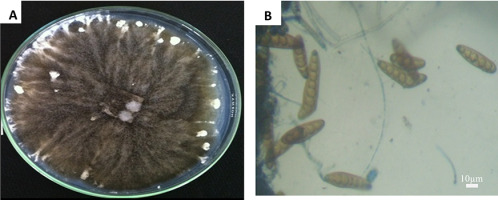
On the rice flour-based medium, fungus isolates formed fluffy and cottony aerial mycelia. The colony was gray to dark greenish gray in color (Figure 1); the conidiophores grew in singles or in groups, branched or simple, multiseptate, flexuous, sometimes with geniculate upper part, brown to black. The conidia of the fungal species were straight, cylindrical, usually curved, light brown to golden brown, with six to 14 transverse partition. The conidial size ranged from 63 to 153 (avg. 109) µm × 14 to 22 (avg. 17) µm (Figure 1B). Morphologically, this fungus was therefore identified as B. oryzae (Ellis, 1971; Ou, 1985; Tarr, 1962).
Pathogenicity
Inoculation of Typha latifolia Leaves With Spore Suspension
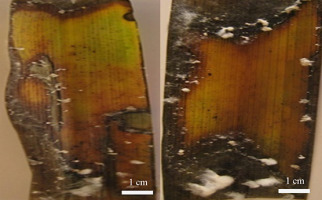
The size of the lesions induced by B. oryzae varied depending on the isolate. The four isolates of B. oryzae (Hor1, Hor2, Hor3, and Hor4) caused lesions of different sizes on T. latifolia (14.4, 12.3, 15.8, and 13.8 mm, respectively) (Table 2, Figure 2).
Inoculation of Typha latifolia Leaves With Mycelial Discs
Table 3 shows that B. oryzae isolates Hor4 and Hor2 induced fairly large lesions (20.8 and 16.7 mm, respectively) on leaves of T. latifolia, compared to those induced by Hor3 (11 mm) and Hor1 (9.2 mm) (Figure 3).
Sporulation of Bipolaris oryzae Isolates
Through inoculation with either mycelial discs or spore suspensions, B. oryzae was able to sporulate on T. latifolia leaves, and no significant difference in intensity between the isolates tested was observed. Indeed, the average number of conidia ranged between 0.13 × 105 conidia/cm2 and 0.83 × 105 conidia/cm2 for the first technique (Table 4), and from 0.1 × 105 conidia/cm2 to 0.6 × 105 conidia/cm2 for the second technique (Table 5).
Table 4
Sporulation of Bipolaris oryzae on Typha latifolia leaves after inoculation with mycelial discs (×105 spores/cm2).
| Bipolaris oryzae isolates | Hor1 | Hor2 | Hor3 | Hor4 |
|---|---|---|---|---|
| Number of spores | 0.76 a | 0.83 a | 0.13 a | 0.2 a |
Inoculation of Rice Varieties With Conidial Suspension
After 7 days of inoculation, all the rice varieties tested displayed high sensitivity towards the four isolates of B. oryzae studied, as reflected by necrotic areas on the inoculated leaf surfaces. Thus, similar symptoms were observed in the five varieties of rice; they were generally of reddish-brown color, oval shape surrounded by a pale yellow halo, and were relatively uniform and regularly distributed (Figure 4).
Figure 4
The symptoms of rice leaves artificially inoculated with spore suspensions of Bipolaris oryzae; (A) Taibonet variety, (B) Eurano variety, and (C) Cererrer variety.

As shown in Table 6, among the five rice varieties tested, Elio was the most sensitive to the Hor1 isolate, with a severity index of 90.73%, followed by the varieties Taibonet, Cererrer, and Eurano, whose severity indexes reached 85.18%, 79.63%, and 79.62%, respectively. In comparison, the Arpa variety was less sensitive, showing a severity index of 35.18% against the same isolate.
The Cererrer and Elio varieties were more sensitive to the Hor2 isolate, with a severity index of 90.74% and 81.47%, respectively, which was superior to those of the Taibonet, Eurano, and Arpa varieties, which did not exceed 74.99%. The sensitivity of the Cererrer variety to the Hor3 isolate was marked by a high severity index of 90.73%, followed by the Elio, Eurano, Taibonet, and Arpa varieties, with respective indexes of 79.63%, 74.07%, 69.44%, and 62.03%. The Cererrer and Elio varieties were the most sensitive to the Hor4 isolate, showing the highest severity index (92.59%). However, the severity varied between 87.03% and 59.26% for the varieties Taibonet, Eurano, and Arpa.
Table 6
Mean percentages of disease severity induced by Bipolaris oryzae isolates on rice varieties.
Table 7 shows that for a given isolate, the severity varies depending on the inoculated rice variety. The Hor4 isolate was found to be most pathogenic to the Cererrer and Elio rice varieties, whose disease severity index reached 92.59%, followed by the Taibone (87.03%) and Eurano (84.25%) varieties.
The Hor3 isolate was highly pathogenic to the Cererrer variety (90.73%) and less pathogenic on Elio and Eurano varieties, with respective disease indexes of 79.63% and 74.07%. The severity indexes for the Hor2 isolate were the highest in the Cererrer and Elio rice varieties (90.74% and 81.74%, respectively), but did not exceed 74.99% in the Eurano, Taibonet, and Arpa varieties. Hor1 was more pathogenic to the Elio and Taibonet varieties, with respective indexes of 90.73% and 85.18%.
Table 7
Mean percentages of disease severity induced by Bipolaris oryzae isolates in rice varieties.
The sporulation ability of B. oryzae isolates from T. latifolia on the leaves of five varieties of rice showed pronounced variability between isolates. The highest spore number of the Hor1 isolate was observed in the Eurano variety, with 1.16 × 105 spores/cm2, followed by the Arpa and Taibonet varieties, with 0.26 × 105 and 0.16 × 105 spores/cm2, respectively. However, the sporulation was very low in the Elio and Cerrerer varieties, in which the number of spores was reduced to 0.06 × 105 spores/cm2 (Table 8).
The highest number of spores of the Hor2 isolate was 1.06 × 105 and 1.03 × 105 spores/cm2 in the varieties Elio and Arpa, respectively, followed by that in the Cererrer and Eurano varieties, with 0.23 × 105 and 0.13 × 105 spores/cm2, respectively. The Taibonet variety showed the lowest spore density, equal to 0.06 × 105 spores/cm2 (Table 8).
The sporulation intensity of the Hor3 isolate on the leaves of the Arpa and Cererrer varieties was high (1.43 × 105 and 1.33 × 105 spores/cm2, respectively). In comparison, on the leaves of the Eurano and Elio varieties, the spore number was 0.7 × 105 and 0.63 × 105 spores/cm2, respectively. Moreover, no spore was found on the leaves of the Taibonet variety (Table 8).
The maximum spore production of the Hor4 isolate was observed on the leaves of the Eurano variety, with 2.26 × 105 spores/cm2. However, no spore was found in the other varieties (Taibonet, Arpa, Cererrer, and Elio).
Table 8
Sporulation of the four isolates of Bipolaris oryzae on the leaves of the five varieties of rice (×105 spores/mL).
. Discussion
In the pathogenicity tests, Moroccan isolates of B. oryzae, which were isolated for the first time from the leaves of T. latifolia, were revealed to be pathogenic on rice seedlings, as indicated by necroses and discoloration on a large portion of the leaf blades of the five studied varieties. We can consider T. latifolia an alternative host of B. oryzae. Our result is in agreement with previous research results indicating that B. oryzae had a wide range of hosts, including Oryza sativa, Triticum aestivum, Panicum virgatum, Zea mays, Brachypodium distachyon (Farr & Rossman, 2017; Kaspary et al., 2018; Manamgoda et al., 2014), and Typha orientalis (Wang et al., 2019). Based on the severity index, B. oryzae isolates showed variable pathogenic capacity to the five tested rice varieties. The high pathogenicity of B. oryzae on rice varieties is supported by Monira et al. (2021), who reported that B. oryzae can attack rice seedlings and seeds and can remain viable for up to 5 years on Heera 2 hybrid rice seeds under suitable storage conditions. Based on their response to B. oryzae inoculum, the tested varieties were classified as moderately sensitive to highly sensitive according to the severity scores defined by Boka et al. (2018).
Regarding the sporulation ability, the tested isolates of B. oryzae succeeded in producing abundant spores on the leaf lesions, suggesting successful infectivity irrespective of rice variety and host. The attack of a plant by a pathogen depends on the pathogen itself (Notteghem et al., 1980) or on the host plant genotype (Marchetti & Bonman, 1989). However, the severity scores can vary depending on the isolates and rice variety. Similar observation was made by Ouazzani Touhami et al. (2000), who found different pathogenicity levels among isolates of Helminthosporium spiciferum and H. australiensis, whose capacity to sporulate on host plants also depends on the rice variety.
Helminthosporium can be isolated from a wide range of hosts, including corn and grasses (Nelson & Kline, 1961, 1962; Nelson et al., 1963). The parasitism of B. oryzae on corn was reported by Ou (1972) and Vidhyasekaran et al. (1986). The lesions appeared at approximately 18 hr after the inoculation of B. oryzae on the rice plant (Dallagnol et al., 2009). The results showed that the pathogenicity of B. oryzae was not specific to the rice from which it was isolated. This fungus can, without doubt, extend to other species widely cultivated in the vicinity of rice fields. Serghat, Ouazzani Touhami, & Douira (2005) have, in fact, shown that B. oryzae can be isolated from grasses, such as wheat, corn, and barley, that are located close to rice fields. The fungi present on leaf lesions in weeds can constitute a potential source of inoculum for rice plants. The presence of these weeds in and around rice fields helps maintain a high level of contaminating inoculum, promoting the progression of the epidemic. Indeed, the production of secondary inoculum via multiplication of infectious elements on the leaf lesions generates new contaminations and allows the disease to progress in rice fields (Serghat, Mradmi, et al., 2005; Serghat, Ouazzani Touhami, & Douira, 2005). According to Boulet and Bouhache (1990), the presence of an adventitious flora adapted to the conditions of rice fields, such as Echinochloa crus-galli and E. phyllopogon, greatly compromises the health of rice fields. Likewise, this flora harbors the same fungi that are found on rice leaf lesions. In the same context, the studied mycoflora on Echinochloa phyllopogon and Phragmites australis (two weeds adapted to rice fields) showed two types of fungi: true rice pathogens (Pyricularia grisea, Helminthosporium oryzae, H. sativum, H. australiensis, H. spiciferum, and Curvularia lunata) and saprophytes that cause rice discoloration (Trichoderma harzianum, Alternaria alternata, Nigrospora oryzae, Epicoccum nigrum, Fusarium moniliforme, Cladosporium herbarumand, and Trichothecium roseum) (Serghat, Mradmi, et al., 2005). Weeds in rice fields can also harbor other pests, including viruses, bacteria, and insects. Thus, in the presence of Typha sp., the development of Sesamia (Sesamia nonagrioides) in rice fields is much faster because this weed species is a plant host for the first larval stages of this predatory rice insect (Fazeli, 1992). Echinochloa crus-galli was identified as capable of hosting and transmitting the southern rice black-streaked dwarf virus in South China (Li et al., 2012). In addition, E. crus-galli has proven to be an important reservoir of Aphid and Barley yellow dwarf luteovirus (BYDV) (Geissler & Karl, 1989). According to Bouhache et al. (1989), weeding rice fields and eradicating the surrounding weeds may protect rice plants from infection by the inoculum produced on these weeds. Our data provide important information on the novel isolates of B. oryzae and on the possibility of latent inoculum transmission that leads to disease in crops and weeds surrounding rice fields. However, the development of pathogen control strategies based on the genetic structure of the pathogen populations would certainly be effective (Nagaty & El Assal, 2011), and more knowledge is needed on host shifting and host expansion in fungal plant pathogens.