. Introduction
Sarcosoma globosum (Schmidel) Casp. (witches cauldron), in the order Pezizales (Samuelson et al., 1980), is primarily associated with old-growth boreal spruce (Picea spp.) dominated forests of northern Europe (e.g., Finland, Russia, and Sweden) with sporadic occurrences in central Europe (Dahlberg, 2015; Nitare, 2009; Ohenoja et al., 2013; Ruotsalainen et al., 2023; Vabuolė et al., 2024). In 2015, S. globosum was classified as near-threatened (red-listed in ten European countries) by the International Union for Conservation of Nature (ICUN) primarily due to logging activities that resulted in the decline and degradation of its habitat (Dahlberg, 2015). Rare occurrences of S. globosum have been reported in eastern parts of North America (Bazzicalupo et al., 2022; Dahlberg, 2015), but it has been suggested that these may have been misidentified (Dahlberg, 2015). In the scientific literature, there are no known occurrences of S. globosum documented in western North America (e.g., Dahlberg, 2015; Kroeger & Berch, 2017). However, in 2021 citizen scientists (e.g., members of the general public without formal training) reported the presence of S. globosum in north-central British Columbia, Canada’s most western province (CBC, 2021). Thus, the species has been identified as requiring further study in Canada (Bazzicalupo et al., 2022; Vabuolė et al., 2024). Information on fungal distributions is essential to aid in their conservation, especially under a changing climate and land cover change (Tedersoo et al., 2022; Větrovský et al., 2019).
Citizen scientists play a large role in advancing mycology and our knowledge of species distributions (Bazzicalupo et al., 2022). Sarcosoma globosum is a good candidate for a citizen science survey as it is an easily recognizable fungus and was recently used as a model species in a Finnish study to document the increase of citizen scientist fungal observations due to an increase in digital platforms (i.e., cell phone applications; Ruotsalainen et al., 2023). The species produces distinctive gel-filled sporocarps, normally 5–10 cm in diameter, that resemble a barrel or cauldron that typically emerge during the spring melt and can be found between April and June, being later in the season at latitude increases (Beug et al., 2014; Martinsson & Nitare, 1986; Ohenoja et al., 2013). Emergence coincides with particularly warm and wet springs, at least in the drier areas of its range (Zvyagina, 2015). However, even within these bounds, fruiting events of S. globosum are highly variable (Semaškaitė et al., 2020). For example, no fruiting was recorded in the middle taiga zone of Siberia, Russia between 2009–2012 (Zvyagina, 2015), and only 36% of known fruiting sites in Uppsala County, Sweden produced sporocarps in 2020 (Blomqvist, 2020). However, individual sites may produce anywhere from 0–926 sporocarps (Semaškaitė et al., 2020). This variability may enhance the perception of the species’ rarity, as occurrences may be missed in years where they do not fruit.
Another reason for its suitability for a citizen science survey is that S. globosum has no known lookalikes (Ruotsalainen et al., 2023). However, in North America there remains a misconception that S. globosum could be confused with S. latahense or S. mexicanum, but both species have different morphology, are represented in different phylogenetic clades, and are now accommodated under the names Pseudosarcosoma latahense and Urnula padeniana, respectively (Carbone et al., 2013a, 2013b). Indeed, the genus Sarcosoma is only represented by a single sequence of its type species S. globosum (Carbone et al., 2013a). The most commonly chosen and easily amplified genetic marker in mycology is the nuclear ribosomal internal transcribed spacer (ITS) region (Lücking et al., 2020; Schoch et al., 2012). Several ITS sequences for S. globosum have been deposited in GenBank (National Center for Biotechnology Information, 2024) allowing for phylogenic comparison between specimens observed in Europe with those found in North America.
Sarcosoma globosum is strongly associated with spruce, being found primarily in mature, spruce-dominated forests with no history of clearcutting (Blomqvist, 2020; Ohenoja et al., 2013; Vabuolė et al., 2024; Zvyagina, 2015). Observations from young or disturbed stands are comparatively rare, but a recent assessment of S. globosum’s habitat and distribution in Lithuania observed the species in a large proportion (ca. 25%) of young Norway spruce (P. abies) stands of cultural origin (i.e., planted; Vabuolė et al., 2024). While it appears that spruce must be present for S. globosum to survive, it does not have to be the dominant species, and observations have been recorded in mixed-conifer woodlands (Vabuolė et al., 2024) or in areas such as over-mature aspen forests in Siberia (Zvyagina, 2015). However, observations of S. globosum decline in areas where pine (Pinus sp.) replaces spruce (Blomqvist, 2020). Within its documented range, the fungus has been observed on acidic and calcareous soils, especially in areas where the soil remains damp throughout the spring and summer, which have intact forest floors typically covered by bryophytes (Ohenoja et al., 2013; Vabuolė et al., 2024; Zvyagina, 2015).
Fungi can be correctly identified at the species level by applying an integrative approach based on the morphological, ecological, and phylogenetic species concepts (Lücking et al., 2020; Xu, 2020). Thus, the aim of this study is to (i) establish that the sporocarps observed in Canada are S. globosum via these three species concepts, (ii) characterize the distribution of S. globosum in British Columbia using a citizen science survey, and (iii) summarize the distribution of S. globosum across Canada using publicly available databases.
. Material and methods
Citizen science survey
A four-year (2021–2024) citizen science survey was undertaken in the region of Prince George, British Columbia, Canada. The survey was promoted through national (Canadian Broadcasting Corporation) and local news agencies/websites (e.g., Prince George Citizen and Facebook). The mean annual air temperature in the region from 1991–2020 is 4.3 °C (the hottest month is July with a mean of 15.9 °C and the coldest month is December with a mean of −6.7 °C), the mean annual precipitation is 590 mm with 433 mm of rainfall and 203 cm of snowfall, and a mean of 120 days with snow cover (Environment Canada, 2024). An online form was created where the general public could document the GPS location, date, and habitat of their observations of S. globosum. Instructions were provided on how to obtain a GPS location using cell phone apps. Observers were also asked to submit photos of the sporocarp morphology as well as the habitat in which they were observed. These photos allowed for species identification based on morphology in areas of the province where mycologists could not easily travel. Unverified observations (i.e., those with no photos or specimens) were still recorded in our data as S. globosum is easily recognizable, but we have labeled the data as unverified in our results. Specimens were collected from the region (n = 125) and identified based on their macro- and micromorphological characteristics (Beug et al., 2014; Martinsson & Nitare, 1986). The sporocarp is gelatinous, dense and heavy containing a substantial amount of water when fresh. They typically measure 3–12 cm in diameter, 3.5–7 cm in height and exhibit irregularly rounded shapes. The outer surface of these sporocarps appears brown and is fibrous-tough, elastic, deeply wrinkled, and folded. The hymenophore is located in a depression on the upper portion, is saucer-shaped, and is blackish to brown in color. Asci are cylindrical, 8-spored, and 250–500 × 12–15 µm; spores are 20–30 × 7–12 µm, elliptic, smooth, uniseriate, hyaline, asci cylindric to subcylindric, paraphyses slender, and brown in color. Microscopic features were observed under a light microscope.
Molecular and phylogenetic analysis
Mycologists collected two S. globosum specimens from British Columbia in western Canada (May 2022, accession numbers: OP023316, PP922187; University of Northern British Columbia (UNBC) herbarium PRS-2022-21 – one specimen was too damaged following sampling to be added to the herbarium) and one specimen from New Brunswick in eastern Canada (May 2020, accession number OR529395; New Brunswick Museum herbarium NBM-F-009811). The sequences were analyzed in three different laboratories, and the general protocol was followed. A Fungi/Yeast Genomic DNA Isolation Kit (Norgen BioTek Corp., St. Catharines, Ontario, Canada) was used following the manufacturer’s protocol with slight modification to extract fungal DNA from the hymenium. Bead beating was carried out for 2 min at 1,500 strokes per minute in a SPEX 2000 Geno/Grinder (ATS Scientific, Burlington, Ontario, Canada).
The ITS gene was chosen to be consistent with the sequences available in GenBank. The full ITS region was sequenced by amplifying the ITS1, the 5.8S ribosomal RNA gene (5.8S), and the ITS2 using the forward primer ITS1-F_KYO1 (Toju et al., 2012) and the reverse primer LSU476A-R (Asemaninejad et al., 2016; accession number: OP023316) or ITS4_KYO1 (Toju et al., 2012; accession number: PP922187). The PCR reaction volume of 25 µL contained final concentrations of 0.2 µM for both primers, 3.4 ng/µL of fungal genomic DNA, and a 1x concentration of multiplex master mix (Qiagen, Toronto, Ontario, Canada). Amplification of the PCR fragment was performed with the following thermal cycler (Bio-Rad C1000 Touch) conditions: initial denaturation at 95 °C for 15 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 57 °C for 90 s, extension at 72 °C for 90 s, and a final extension at 72 °C for 10 min. The PCR product was purified using a PCR Purification kit (Norgen BioTek Corp., St. Catharines, Ontario, Canada) following the manufacturer’s protocol.
The amplified DNA was sequenced bidirectionally through Sanger sequencing (Applied Biosystems 3130x/Genetic Analyzer; accession numbers: OP023316, OP023316) or long-read sequencing (Pacbio; accession number: PP922187). The amplicon target was too large for a single primer set to be used on the Sanger instrument. Thus the following primers were used; forward primers ITS1-F_KYO1 (Toju et al., 2012), ITS3 (White et al., 1990) and LSU200A-F (Asemaninejad et al., 2016) with the reverse primers ITS2_KYO1, ITS4_KYO1 (Toju et al., 2012), and LSU476A-R (Asemaninejad et al., 2016). Sequences were analyzed and assembled using SnapGene software (version 6.1) and submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank).
For phylogenetic tree construction, sequences within the family Sarcosomataceae and sequences of S. globosum were collected from GenBank following Carbone et al. (2013a). For reference, additional sequences of P. latahense, S. orinetale, and U. padeniana were included in this analysis. These sequences were then aligned in MEGA 11.0.13 using the ClustalW application (Tamura et al., 2021). Following alignment, sequences were manually corrected with final alignments uploaded in TreeBase (study number S30669). Sequences were then implemented within MrBayes (3.2.7) (Huelsenbeck & Ronquist, 2001), where Bayesian analysis was performed (General time reversible, 2 runs, 8 chains, inverse gamma rates, sampling every 100th generation, temperature = 0.1) until adequate convergence (average standard deviation of split frequencies < 0.01) was obtained after 1M generations (Ronquist et al., 2011). This tree was implemented within MEGA 11.0.13, where maximum likelihood analysis was performed on the tree (500 bootstraps, general time reversible model, subtree pruning regrafting fast [SPR level 3]). Significance thresholds were above 70% for maximum likelihood bootstrapping (BP) values and 99% for posterior probability (PP).
Database search for Canadian observations
Records of S. globosum across Canada were obtained from the Mycology Collections Data Portal (Mycoportal, 2024) and the Global Biodiversity Information Facility (GBIF, 2024). Both databases contain species observations from a network of data sources from herbariums at universities and museums, iNaturalist, Mushroom Observer, as well as agencies that provide taxonomic, environmental, and specimen-based information. Care was taken not to include duplicated information.
. Results
Morphology and phylogenetic analysis
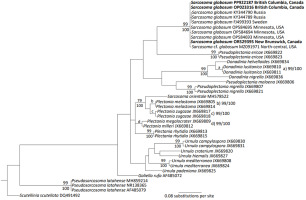
The morphology of the collected specimens was consistent with the taxonomic description for S. globosum (Section 2.1; Figure 1). The ITS region of specimens collected from British Columbia (accession number: OP023316 length: 958 bp, accession number: PP922187 length: 604 bp) and New Brunswick, Canada (accession number: OR529395 length: 631 bp) shared 100% sequence identity. These Canadian specimens had sequence identities between 99.7 and 100% with S. globosum sequences in the GenBank database collected from Russia and Sweden as well as Minnesota, USA. Both the Bayesian and maximum likelihood models show that the specimens acquired from British Columbia and New Brunswick significantly group with the S. globosum sequences found in Europe and the USA (Figure 2). The apparent grouping of the Swedish and Russian sequences was not significantly different from the other S. globosum sequences. The Russian and Swedish samples had a sequence identity of 99.8%. A saxicolous lichen species identified as S. cf. globosum and obtained from north-central USA (Healy et al., 2022) also significantly grouped with the European and North American S. globosum sequences with a sequence identity of 98.8% and 99.4%, respectively.
Figure 1
Sporocarps of Sarcosoma globosum observed in spruce-dominated habitat collected at (A) Aleza Lake Research Forest, British Columbia, Canada (Photo: M. D. Preston, May 2022), (B) Glassville, New Brunswick, Canada (Photo: A. Justo, May 2020), (C) sporocarp observed in the laboratory (Photo: M. D. Preston, May 2022), and (D) dissected sporocrap revealing gelatanous interior (Photo: A. Justo, May 2020).

Figure 2
Consensus tree resulting from Bayesian ITS analysis of Sarcosoma globosum and relatives within the Sarcosomataceae family. Nodes with maximum likelihood BP (above) and Bayesian PP (below) supported by both analyses were annotated. Bold indicates the sequences represented within this study. The location of the S. globosum sample collection is indicated.
Observations in British Columbia
Most S. globosum observations were documented in 2021 and substantially declined in subsequent years in British Columbia (Figure 3). The vast majority of observations were documented around Prince George, British Columbia, and comprised an area of approximately 62,000 km2 (Figure 4). These observations were primarily recorded in sites that had no formal protection and were situated on private property or within areas designated for future forestry activity (e.g., forestry cutblocks) or development. Only two verified observations at the Aleza Lake Ecological Reserve and three unverified observations within the Giscome Portage Trail Protected Area and Purden Lake Provincial Park were located within protected areas.
Figure 3
Total number of Sarcosoma globosum observations in Canadian records. Data are provided in different intervals due to low observation number. Verified and unverified data represent our citizen science survey, other data were obtained from publicly available records (GBIF, 2024; Mycoportal, 2024).
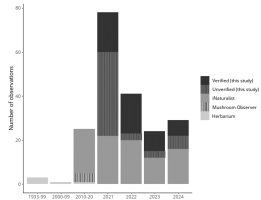
Figure 4
Verified and unverified observations of Sarcosoma globosum in British Columbia from 2021–2024. Specific focus on Prince George and Aleza Lake Research Forest. Map created using QGIS (Creative Commons Attribution-ShareAlike 3.0 license (CC BY-SA)) using Atlas of Canada base data (Open Government License – Canada – Version 2.0).
All observations occurred in the presence of spruce (Picea spp.) and usually in spruce-dominated forests (Figure 5A). Other tree species, such as birch (Betula papyrifera), cedar (Thuja plicata), and subalpine fir (Abies lasiocarpa), were often present and occasionally dominant over spruce. Observations were typically documented close to a stream, river, or lake and were reported in clusters of sporocarps (ca. 5–10). Rarely were single sporocarp observations submitted.
Figure 5
(A) Typical spruce (Picea sp.) dominated forest habitat in north-central British Columbia, (B) experimental research plot at Aleza Lake Research Forest that was plowed prior to planting with spruce, (C) clusters of Sarcosoma globosum observed in the experimental research plot, and (D) S. globosum observed growing at the base of a spruce (Photos: M. D. Preston, May 2022).

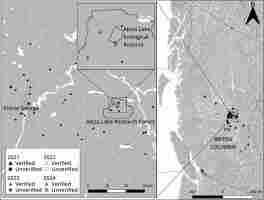
Observations from the Aleza Lake Research Forest (Figure 4) are particularly notable. Several hundred individual S. globosum sporocarps were observed in a highly disturbed experimental plot that was previously forested, plowed level following harvesting, and then planted with spruce trees in the 1950s (Figure 5B–C). Sporocarps were also observed at the base of several spruce trees (Figure 5D), and notably fruiting in a line along a decayed, moss-covered spruce log on the forest floor (Figure 6).
Observations across Canada
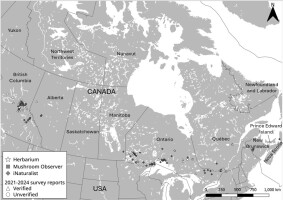
Sarcosoma globosum has been recorded in collections in Ontario and Québec from the 1930s–1990s, New Brunswick in 2009 and 2020, and British Columbia from 2021–2023 (this study; Table 1). Where data exists, these specimens were observed in the presence of spruce. Due to national media attention, our citizen science survey also received observations from other Canadian provinces, including Alberta, Ontario, and Québec (Figure 7). Unverified records from iNaturalist show S. globosum has been observed across Canada, specifically (from east to west) in Nova Scotia, New Brunswick, Québec, Ontario, Manitoba, Alberta, and British Columbia (Figure 7). As of June 2024, there were 99 observations recorded in public databases, and of these observations, only 12 were within protected park boundaries, all of which were observed in Ontario (Algonquin Provincial Park; Pukaskwa National Park; Quetico Provincial Park; Slate Islands Provincial Park; and Sleeping Giant Provincial Park), except for Wells Gray Provincial Park that is in British Columbia.
Table 1
Records of Sarcosoma globosum in Canadian collections. Data from mycoportal.org (accessed June 2024) and collections made by the authors.
. Discussion
This study presents the first documented occurrence of S. globosum in British Columbia, expanding the known distribution of the species to western Canada, having been previously reported in northern Europe, Asia (Siberia), and eastern North America (Dahlberg, 2015; Nitare, 2009; Ohenoja et al., 2013; Ruotsalainen et al., 2023; Vabuolė et al., 2024). Specimens matched the morphological description for S. globosum and were consistently observed in spruce-dominated habitats in spring and early summer, consistent with observations in Europe (Ohenoja et al., 2013; Ruotsalainen et al., 2023; Vabuolė et al., 2024). The sequence identity of the North American and European samples (99.7%) and the Russian and Swedish samples (99.8%) exceeds even narrow identity thresholds for species identification (98.5%; Lücking et al., 2020). These exceedingly small differences between sequences can be attributed to intragenomic variation with the ITS region brought about by a point mutation in one of the multiple copies in the genome during cell replication (Lücking et al., 2020). Carbone et al. (2013a) identified the genus Sarcosoma as an independent clade within the Sarcosomataceae, which was only represented by a single sequence of S. globosum. Our phylogenetic analysis adds nine new S. globosum sequences, which all significantly group with this original sequence. It is also worth noting that we are not aware of any other biogeographical study of S. globosum in the literature that has applied phylogenetic analyses. By combining morphological, ecological, and phylogenetic analysis we were able to demonstrate that specimens collected in eastern (New Brunswick) and western (British Columbia) Canada are the same S. globosum as reported in Europe.
Due to the dedication of citizen scientists, S. globosum was consistently observed over a wide geographic area (62,000 km2) in all four consecutive years of our survey, suggesting the species is not rare in British Columbia. The majority of observations came in 2021 due to large media interest that raised the profile of the species to the general public. Fewer observations after year one should firstly be attributed to reduced public awareness and survey fatigue rather than solely a result of fewer sporocarps on the landscape. However, it is highly likely that there is annual variation in S. globosum numbers in British Columbia, as this was recently demonstrated in a multi-year study in Finland on the same species (Ruotsalainen et al., 2023). While most observations were in small clusters (<10 sporocarps), the Aleza Lake Research Forest consistently produced several hundred sporocarps at multiple locations within the forest in every year of the survey. Suggesting that, at least for the Aleza Lake Research Forest, 2021–2024 were ideal years for sporocarp formation. Future citizen science surveys should aim to promote the survey every year it is conducted to keep the public engaged. We also recommend using applications such as iNaturalist as these are user-friendly for the general public while providing GPS locations and photos for verification. Ruotsalainen et al. (2023) reported that digital platforms have been shown to increase citizen-based observations beyond traditional environmental authorities. The authors also caution that these changes in observation activities may disguise a true change in fungal populations. Nevertheless, these digital tools are promising for the detection of species in large and relatively un-populated areas (compared with Europe), such as northern British Columbia.
Sarcosoma globosum appears to have a distribution across Canada (British Columbia to Nova Scotia), having been documented in Canadian herbariums since 1933 and in public databases since 2010. While no genetic information exists for the majority of these specimens, it is probable they are S. globosum based on their morphology and their collection/identification from spruce-dominated habitats. While speculative, the observation of similar ITS sequences collected on opposite sides of Canada (ca. 4,000 km apart) in British Columbia and New Brunswick and in the midwestern US state of Minnesota near the Canadian Provinces of Manitoba and Ontario, suggests specimens observed in between these observations likely have the same ITS sequence. However, this hypothesis should be tested.
There is still no consensus in the literature on whether S. globosum is a saprotroph or mycorrhizal fungus, which has been speculated due to its association with spruce (Dahlberg, 2015; Nitare, 2009), and this lack of knowledge hinders its conservation. Our observation of sporocarps on a rotten spruce log (Figure 6) suggests S. globosum plays an important role in the nutrient transfer from decaying wood to the soil and vice versa. However, it does not confirm its ecological role, as some mycorrhizal species are known to produce fruiting bodies in decaying wood (Stokland et al., 2012). Evidence from stable isotope analyses shows that nitrogen can be actively transferred from the soil to decaying wood by both saprotrophic (Philpott et al., 2014) and mycorrhizal fungi (Mäkipää et al., 2017). Mineral nutrients obtained from the wood by these fungal guilds could then be translocated through the forest via their respective hyphal networks (Boddy & Watkinson, 1995; Lindahl & Tunlid, 2015). This implies that S. globosum may play an important role in nutrient fluxes throughout the forest ecosystem and future studies should examine its ecological role using isotope studies and by sequencing its complete genome (Li et al., 2018). Intriguingly, a saxicolous lichen sequence identified as S. cf. globosum and obtained from north-central USA (Healy et al., 2022) significantly grouped with other S. globosum sequences, suggesting the species may have several ecological roles, or the lichenized fungus may be an undescribed species of Sarcosoma.
Few S. globosum observations were recorded in protected areas across Canada. The main threat to the species is believed to be habitat loss from logging activities (Dahlberg, 2015; Vabuolė et al., 2024). British Columbia is an intensively logged province (Gilani & Innes, 2020), and this may reduce the amount of available habitat. Encouragingly, S. globosum has been observed in highly disturbed sites, such as a 27-year-old Norway Spruce plantation in Lithuania (Vabuolė et al., 2024), and a 50-year-old plantation at Aleza Lake Research Forest (this study), suggesting the species does not require old-growth forest as previously thought (e.g., Dahlberg, 2015).
Climate change is likely to impact the distribution of S. globosum by primarily impacting the health of spruce forests. Tree mortality rates have been increasing due to water deficits, pest-outbreaks (e.g., spruce bark beetle), and increased frequency of storms (Chavardès et al., 2013; Daniels et al., 2011), which result in vegetation change (Reid et al., 2022). Further, warmer conditions are leading to drier forests with an increase in forest fire frequency (Baltzer et al., 2021). Together, these changes could destroy or radically alter the forest composition, making it unsuitable for S. globosum habitat.
Overall, our citizen science survey and data from public databases support the current Canadian national conservation status of ‘Apparently Secure/Secure’ (N4, N5) (CESCC, 2022) and indicate that S. globosum should occur in Canada where suitable habitat is found. That there are no historical records in British Columbia is probably due to limited mycological surveys because of the geographical size of British Columbia (ca. 945,000 km2; Kroeger & Berch, 2017) rather than the species being a new introduction. While this study expands the known Canadian distribution of S. globosum, its true geographical extent across North America remains unknown. A more detailed investigation should be conducted in the USA, examining the numerous records in herbaria and publicly available databases (e.g., Mycoportal, 2024). In northern Canada, further surveys should be conducted in the spruce-dominated forests of the Northwest Territories and the Yukon (CFS, 2022). However, citizen science surveys in these areas will be more challenging as their combined population is ca. 90,000 compared with the Canadian population of ca. 41 million (Statistics Canada, 2024).
. Conclusions
This study documents the presence of S. globosum in British Columbia and the fact that the species appears to be found throughout Canada, where spruce-dominated forests occur. Importantly, we confirm that S. globosum found in Canada is the same species observed in northern Europe. Sarcosoma globosum does not appear to be at imminent risk of extirpation in Canada, but climate change, forest fires, and logging activities may limit its geographical distribution in the future. This study provides evidence to evaluate the global conservation status of S. globosum, but more extensive surveys are still required to understand its North American and global distribution better.