Introduction
The first mushrooms appeared on the earth’s surface as early as 800 million years ago, much earlier than the first representatives of the animal and plant kingdoms (Bonneville et al., 2020). Currently, it is estimated that there may be more than 5 million species of mushrooms; to date, more than 100,000 species have been described (Bruns, 2006). The development of molecular genetics in the twenty-first century made it possible to determine the relationship between different groups of mushrooms. However, many taxonomic groups still have inaccurate connections. Mushroom taxonomy is still evolving, and changes are taking place at the family as well as phylum and division levels. One major international study involving detailed phylogenetic analyses has divided mushrooms into the Dikarya subkingdom, which comprises the two most numerous divisions, Ascomycota and Basidiomycota, and various other divisions and subdivisions (Hibbett et al., 2007). The term “mushroom” is itself not a taxonomic category but is used to refer to fruiting bodies that appear below (hypogeous) or above (epigeous) the ground of the phyla Basidiomycota and Ascomycota from the Dikarya subkingdom (Ganeshpurkar et al., 2010).
Mushroom fruiting bodies have been part of the human diet at least since the Neolithic period. This is indicated by archeological excavations dating back to 8000 BC, whereas the first documented therapeutic use can be associated with the finding of a frozen body of a Paleolithic man from 3300 BC, which was discovered in the alpine glacier in the 1990s. The fruiting bodies of the tinder fungus (Fomes fomentarius) and the birch polypore (Fomitopsis betulina), which can be used as provisional wound dressings owing to their lymphatic, anti-inflammatory, and antibacterial properties, were found by the body (Schlegel et al., 2000; Wangun et al., 2004).
More than 2,000 species of mushrooms are considered edible, and the medicinal or health-promoting properties of approximately 700 species of mushrooms have been documented. The most studied pro-health substances contained in mushroom fruiting bodies, mycelia, and spores are polysaccharides, mainly β-glucans. Their effectiveness in the prevention and treatment of diseases has been proven; they are immuno-stimulants, owing to which they have a positive effect on the health of patients with cancer and viral or bacterial diseases (Manzi & Pizzoferrato, 2000). To date, a number of β-glucans extracted from Basidiomycota fungi have been analyzed; the best known include shizophyllan (Schizophyllum commune), pleuran (Pleurotus ostreatus), lentinan (Lentinula edodes), and krestin (Trametes versicolor), which is a polysaccharide–protein complex (Villares et al., 2012) (Table 1, Figure 1).
Table 1
Structural features of main biological active polysaccharides isolated from medicinal and culinary mushrooms.
Species and reference in Figure 1 | β-glucan structure | Reference | |
Agaricus bisporus | (1→6) Linking β-ᴅ-glucose as the main backbone with (1→4)-linked α-ᴅ-mannose units | He et al., 2014 | |
Auricularia auricula-judae | β-(1→3)-ᴅ-Glucan with two β-(1→6)-ᴅ-glucosyl | S. Xu et al., 2012 | |
Flammulina velutipes | 3-O-ᴅ-Mannopyranosyl-L-fucopyranosyl, α-ᴅ-mannopyranosyl, and α-L-fucopyranosyl | Smiderle et al., 2008 | |
Ganoderma lucidum | β-(1→3)-Linked ᴅ-glucan; (1→6)-glucan with (1→4) branches at O-4; α-(1→4)-ᴅ-glucopyranosyl and β-(1→6)-ᴅ-galactopyranosyl with branches at O-6 of glucose and O-2 of galactose | ||
Lentinula edodes | (1→3),(1→6)-ᴅ-Polysaccharide; fucomannogalactan of (1→6)-linked α-ᴅ-galactopyranoses branched at O-2 | Carbonero et al., 2008; Palacios, Guillamón, et al., 2012 | |
Pleurotus ostreatus | (1→3),(1→6)-ᴅ-Polysaccharide; α-(1→3)-ᴅ-glucan | Palacios, García-Lafuente, et al., 2012; Synytsya et al., 2009 | |
Schizophyllum commune | (1→3),(1→6)-ᴅ-Glucan | Numata et al., 2006 | |
Trametes versicolor | α-(1–4) And β-(1–3) glucosidic linkages in their polysaccharide moieties | Awadasseid et al., 2017 | |
Figure 1
Medicinal and culinary mushrooms as a source of polisaccharides. (A) Agaricus bisporus; (B) Auricularia auricula-judae; (C) Flammulina velutipes; (D) Ganoderma lucidum; (E) Lentinula edodes; (F) Pleurotus ostreatus; (G) Schizophyllum commune; (H) Trametes versicolor. Photographs by B. Muszyńska, A. Sękara, K. Sułkowska-Ziaja, and P. Zięba.

Another large group of pro-health substances in mushrooms is phenolic compounds, which have antioxidant effects (Asatiani et al., 2011). They exhibit strong anti-inflammatory and anticancer properties; moreover, ethanol extracts from over 100 different mushroom species show strong antioxidant properties. Phenolic acids such as caffeic acid, hydroxybenzoic acid, and protocatechuic acid have been extracted from the fruiting bodies of the most popular culinary species (Siwulski et al., 2014).
Terpenoids, characterized by strong bioactivity, are important compounds contained in the fruiting bodies of Basidiomycota mushrooms. A number of compounds from this group have been isolated. In most cases, they are sesquiterpenoids, and the best known and studied are those extracted from the fruiting bodies and mycelia of the Lingzhi mushroom (Ganoderma lucidum) (Liu et al., 2007). These substances, similar to β-glucans, exhibit a number of pro-health properties; their anticancer, antibacterial, and antiviral activity has been proven (Mothana et al., 2000).
Lovastatin, which can be found in many culinary mushrooms, especially in oyster mushrooms, has an inhibitory effect on the synthesis of endogenous cholesterol, which is harmful to health. Synthetic equivalents of this substance are used in the treatment of hypercholesterolemia, cardiovascular diseases, or strokes (Muszyńska et al., 2010).
More than 100 species of mushrooms are grown worldwide, and cultivation technologies are very diverse, even for the same species. Different growing media are used; however, the base is typically easily accessible and cheap lignin–cellulose waste and different kinds of sawdust and straw, often enriched with protein additives such as bran. The use of atypical and problematic waste, e.g., coffee grounds or cartons, on which some species of mushrooms are successfully grown commercially, is becoming increasingly popular (Stamets, 2011).
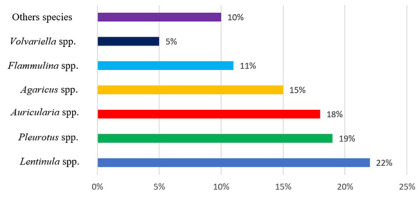
According to Food and Agriculture Organization, mushroom cultivation worldwide in 2018 was approximately 9 million tons. Since 2006, i.e., in just 10 years, there has been a doubling of the volume of fruiting body harvests as well as an increase in the species diversity of cultivated edible mushrooms (Food and Agriculture Organization, 2020). It can therefore be concluded that mushroom production is currently the fastest growing branch of horticulture. The dominant species in cultivation in Europe is the white button mushroom (Agaricus bisporus), the production of which, in 2015, was over 1.1 million tons, accounting for 80% of the total mushroom production on the old continent (Kayzer, 2017). More than a third of the white button mushrooms consumed in Europe are produced in Poland, which is considered the world leader in the export of this species. Currently, approximately 90% of the companies producing edible and medicinal mushrooms operate in China, and the species most commonly grown on a commercial scale belong to the Lentinula, Pleurotus, Auricularia, Agaricus, and Flammulina genera, and account for 22%, 19%, 18%, 15%, and 11% of the world production, respectively (Royse et al., 2017) (Figure 2).
The nutritional and therapeutic use of mushrooms in Asian countries results from centuries of tradition and is much better documented than in Europe.
The technologies for growing these mushrooms have been developed independently in Europe and Asia, with Asian countries having a longer cultivation tradition (using different technologies) and a different production profile compared to Europe and North America. The first attempts to grow mushrooms were made in China in the twelfth century, and they consisted of combining freshly cut tree trunks with those on which the fruiting bodies of the shiitake (Lentinula edodes) already grew (Siwulski et al., 2011). However, the breakthrough was not made until the end of the nineteenth century, when the first pure mycelium culture was achieved by pasteurization. Firstly, composted horse manure was grafted with the obtained clean mycelium of white button mushroom, initiating intensive mushroom cultivation (Szudyga, 1998).
In general, mushroom growing consists of several operations, viz., pure mycelium maintenance, inoculum preparation, substrate preparation, inoculation, incubation, and fruiting. The conditions and technology for each of these operations are specific for mushroom species or even strains (Sánchez, 2010). The spores or clones from wild mushroom fruiting bodies are the source of pure mycelia of the specific mushroom strain, which is produced in specialized laboratories in a sterile environment, and maintained and provided by germplasm suppliers. The commercial inoculum is obtained through the development of selected strain mycelia on cereal grains, e.g., wheat, rye, millet, or synthetic speed spawn. Spawn quality is crucial for the success of mushroom production. Therefore, many studies have focused on modern techniques of best-quality spawn production (Elhami & Ansari, 2008; Nwanze et al., 2005; Pathmashini et al., 2009; Sainos et al., 2006). The optimal composition of the growing substrate for a particular mushroom strain is inoculated and maintained under optimal temperature, moisture, and other conditions for mycelium growth, and then under the conditions that initiate fruiting (Kirbag & Akyuz, 2008; Onuoha et al., 2009).
The agri-food industry produces wastes that are not utilized worldwide, and their disposal is a threat to the environment and public health. Most of these are lignocellulosic materials, which can be suitable substrates for solid-state fermentation processes operated using mushrooms (Ritota & Manzi, 2019). Currently, for 1 kg of edible processed mushrooms, the industry produces 5 kg of spent mycelium substrate and byproducts of mushroom production (Schimpf & Schulz, 2016). Spent mushroom substrate can be used as an organic fertilizer or animal feed. In this context, the valorization of mushroom byproducts into highly valuable compounds can be developed by integrating the potential of these bioactive compounds and their high availability (Buruleanu et al., 2018).
Lentinula edodes
Lentinula edodes is currently the most widely cultivated mushroom in the world. Modern cultivation technologies and traditional methods of cultivation on wood are used in parallel, both in a mass and on a commercial scale. The name “shiitake” refers to the Japanese name of the tree “shii” on which L. edodes is naturally present (Castanopsis cuspidata, Fagaceae) and “take,” which means “mushroom” (Wasser, 2005).
This species is one of the best-researched mushrooms in terms of its pro-health properties, and its effectiveness in treating or supporting the treatment of a number of diseases has been confirmed. The strong immuno-stimulatory effect of the extracts from its mycelium and fruiting bodies is a prerequisite for the effective support of cancer treatment, as well as for combating various bacterial and viral infections. Its most potent active substances are polysaccharides, viz., LEM, isolated only from the mycelium, lentinan, registered as a dietary supplement, and specific substances such as eritadenine or lentysine, which exhibit cardio-protective effects (Bisen et al., 2010). Lentinula edodes is also a valuable source of wholesome protein and B vitamins, as well as macro- and microelements, especially potassium, calcium, zinc, and selenium (Muszyńska, Pazdur, et al., 2017).
The first report on the consumption of L. edodes comes from Japan in 199 BC; the mushroom was a gift to Emperor Chuai. In China, the cultivation of these mushrooms began during the Song Dynasty (960–1127 AD), and in 1313, Chinese writer Wang Cheng described the technology of shiitake cultivation in his Book of Agriculture, paying attention to the choice of the right place and trees (Ito, 1978). During the Ming Dynasty (1368–1644 AD), the physician Wu-Rui wrote in the book Ri Youg Pen Tsao that L. edodes should also be used to treat respiratory diseases, poor blood circulation, and liver problems, as well as to increase life energy. He also wrote about the role of L. edodes in delaying aging. “Shiitake increases life energy, distracts hunger, heals cold and overcomes unstable body energy” (as cited in Rahman & Choudhury, 2012).
The biggest breakthrough in shiitake cultivation was achieved in 1936 in Japan, when K. Kitajima successively produced mycelium of L. edodes on a sterile substrate of straw and then used it for grafting wood. This accelerated the overgrowth of the wood logs and significantly improved the entire production (Mori, 1987). Traditional methods of growing L. edodes on wood logs are still used worldwide, but all-year-round intensive methods that employ artificial logs prepared with heat-treated substrates based on sawdust enclosed in plastic bags are becoming increasingly popular. The so-called “bag-log cultivation” has resulted in a shortening of the growing cycle and an increase in yields, although the final results are highly dependent on the strain, substrate composition, and conditions of incubation and fruiting (Sánchez, 2004). The substrate is a mix of sawdust, grain bran, and CaCO3 with a moisture content of 60%, sterilized at 121 °C for 1 hr (Miles & Chang, 2004). However, intensive cultivation of L. edodes is longer and more complicated than that of other cultivated species. It takes more than 3 months from the creation and inoculation of artificial logs to the first harvest, depending on the strain, which is almost 3 times longer than that of the oyster mushroom. Hence, shiitake mushrooms are much more expensive than oyster or white button mushrooms (Siwulski et al., 2007). Similar to that of all cultivated mushrooms, the yield of L. edodes is related to many factors. The substrate, strain, and proper growing conditions are most important to obtain satisfactory yields for commercial growers (Stamets, 2011). The best way to exhibit the mushroom crop is by indicating the biological efficiency (BE%), which is the weight (kg) of fresh mushrooms harvested from 1 kg weight of dry substrate, expressed in percentages. The acceptable BE% for shiitake commercial growers starts at 40%. However, it is possible to have BE% above 100%. This corresponds to 350–800 g of fresh shiitake mushrooms from a typical 2.5 kg mushroom block, sometimes in more than four flushes (Siwulski et al., 2007).
Pleurotus spp.
There are more than 200 species of oyster mushrooms in the world (Catalog of Life, 2020), and eight species, viz., Pleurotus ostreatus, P. eryngii, P. pulmonarius, P. djamor, P. sajor-caju, P. cystidiosus, P. citrinopileatus, and P. cornucopiae, are commercially grown worldwide (Bellettini et al., 2019). Moreover, there have been many studies on new naturally occurring Pleurotus strains to improve yields. For example, P. albidus is recognized as a novel species for commercial production owing to its high BE% (Lechner & Albertó, 2011). There are a number of technologies for intensive cultivation of these mushrooms, differing in the type of substrate or containers. Oyster mushrooms are one of the easiest to grow because of their rapid growth of mycelia, short production cycle, resistance to diseases, high adaptability to growing conditions, and in consequence, low production costs (Sulistiany et al., 2016).
The first cultivation methods were developed in Germany during World War I and then successfully applied on a large scale, as a result of the search for new sources of food, owing to the hunger problem in Germany. Cultivation consists of inoculating pieces of wood and then harvesting fruiting bodies within a few years until the wood is completely decomposed (Spahr, 2009). However, at the beginning of the 1970s, the cultivation of oyster mushrooms on a commercial scale began, mainly in Asian countries. Several different cultivation technologies have been developed, and new strains have been selected, mainly those with different optimal fruiting temperatures (Sánchez, 2010). The BE% of Pleurotus spp. is highly related to the genus and growing technology. In Poland, most commercial growers use pasteurized wheat or rye straw, which is formed into cubes wrapped in foil, with a weight from 10 to 20 kg. This technology is used for P. ostreatus or P. pulmonarius, and the BE% ranges from 60% to 80%; however, supplementation with bran can result in a BE% above 100% (Gapiński et al., 2001). In bottle technology, used mostly for P. eryngii cultivation in Asia, the substrate contains sawdust of deciduous trees as the main component supplemented with 50% rice bran. The BE% can be above 90%. However, to obtain the highest quality of mushrooms, only two mushrooms are grown from a bottle with one flush; therefore, the BE% is less than 50% (Peng et al., 2000; Yamanaka, 2017). A popular technology in western countries is the cultivation of oyster mushrooms on sterilized substrates made with sawdust (sometimes hardwood pellets) supplemented with bran, placed in a special polypropylene bag with a microfilter, having a weight of wet substrate of approximately 3 kg. The BE% for this type of growing technology is easily above 100% with three flushes (Stamets, 2011).
Pleurotus spp. have a set of enzymes, including carboxymethyl cellulase, xylanase, laccase, and manganese-dependent peroxidase, that decompose a wide variety of lignocellulosic waste (Zhai & Han, 2018). Generally, they can be grown on pasteurized wheat or rice straw; straw of bazar, ragi, sorghum, maize, weed plants, wood, cotton stalks, cotton seed hulls, poplar sawdust, beer grain, coffee grounds, waste paper, walnut and hazelnut shells, palm fruit bunch, etc. can also be used (Das & Mukherjee, 2007; Sözbir et al., 2015; Sulistiany et al., 2016; Yildiz et al., 2002). Pleurotus ostreatus, P. pulmonarius, P. citrinopileatus, P. sajor-caju, and P. cistidiosus are suitable for cultivation with these kinds of substrates (Miles & Chang, 2004).
The growth substrate is commonly a mixture of the aforementioned raw materials, and its composition affects the cultivation time, BE%, yield quantity, and quality (Alananbeh et al., 2014). A recent study revealed that bacteria isolated from fruiting bodies promoted the growth of P. ostreatus (Suarez et al., 2020).
The growth substrate is thermally decontaminated, and spawned with the desired strain in amounts up to 5% of the wet weight of the substrate (Royse, 2002). Pleurotus spp. yield increases with the spawn rate because of faster substrate decomposition and more effective competition with weed molds and bacteria (Sánchez, 2010). The photoperiod of mycelia stimulation for fruiting should be approximately 200–640 lux 8–12 hr a day (M. Ahmed et al., 2013). During fruiting, the strains of Pleurotus spp. produce spores, causing an allergy in some workers; therefore, there is an increase in interest in developing spore-less strains (Obatake et al., 2003). The most popular species for the commercial growth of P. ostreatus can produce 660 million spores per gram tissue per day, which is dangerous for growers, and damages climate systems. The spore-less strain “SPOPPO” of P. ostreatus, protected by CPV Rights and patented by Sylvan Inc., the largest mushroom spawn producer in the world, is now the most popular strain grown worldwide. It was obtained by protoplast fusion, using a wild spore-less mutant that produced deformed fruiting bodies of the commercial strain (Baars et al., 2004).
The nutritional value of Pleurotus spp. depends on the substrate composition. Ali et al. (2007) determined that P. sajor-caju showed maximum fat content when grown on luckrine razing, whereas P. ostreatus showed the same when grown on chimney gutter. Maximum protein was found in the fruiting bodies of P. cornucopiae grown on blow gutter. S. A. Ahmed et al. (2009) showed that a substrate composed of soybean straw produced the highest yield, crude protein, and maximum phosphorus content. The use of paddy straw resulted in higher fiber content, whereas a combination of these substrates produced significantly higher fat, calcium, and iron content. Pleurotus spp. production on wheat bran is recommended to support the market with quality edible mushroom products as well as enable innovative applications of the fermented substrate in animal feeds (Wanzenböck et al., 2017).
Auricularia spp.
The genus Auricularia comprises 10 to 15 species, which are saprophytic, with gelatinous, ear- to shell-shaped fruiting bodies that are recognized worldwide, and with intercontinental to cosmopolitan distributions (Looney et al., 2013). Cultivated species of the genus Auricularia (A. auricula-judae, A. polytricha, A. fuscosuccinea) are of higher economic importance, especially in Asian countries, because of their culinary value and pro-health properties, including antitumor, cholesterol-lowering, anticoagulant, antioxidant, immunomodulatory, anti-inflammatory, and antimicrobial effects (Sękara et al., 2015). In European countries, Auricularia spp. were not seen as edible species; however, they were used in folk medicine as early as the seventeenth century. Herbalist John Gerard described, in 1597, a very detailed use of the wood ear, although he briefly treated the therapeutic value of other mushrooms. John Gerard recommended the wood ear for sore throats, in the form of liquid extract obtained by boiling the fruiting bodies in milk or leaving them immersed in beer, which should then be drunk slowly. The resulting infusion was probably similar to the Chinese soup obtained from the cloud ear fungus, which was also used to treat sore throat. In 1601, Carolus Clusius wrote that Auricularia spp. can be used to treat sore throats, and John Parkinson, in 1640, reported that cooking in milk or soaking in vinegar were the only methods the author knew (Roupas et al., 2012).
Called “Mun” mushrooms in China, the wood ear is the basic ingredient of many Asian dishes, and was probably the first cultivated mushroom in China, based on records in the Chinese Materia Medica from the Tang Dynasty (600 AD), which shows the oldest method of wood ear cultivation. In Asian countries, Auricularia spp. were cultivated as early as the Tang Dynasty (618–907 AD). Chinese pharmacist from the sixteenth century, Li Shih-chen, advised simple technic to cultivate wood ear “… plough the logs with boiled bran, cover them with straw, and the wood ear will grow…” (as cited in Chang, 1977). As with other cultivated mushrooms, the greatest breakthrough was achieved in the 1970s, when intensive cultivation began on sterilized sawdust placed in plastic bags. In 2010, approximately 2 million tons of these mushrooms were produced in China; unlike in Asia, Auricularia spp. crops are marginal compared to other cultivated mushrooms (R. Y. Zhang et al., 2012). Auricularia auricula and A. polytricha are commonly grown on natural logs of deciduous trees, or on a substrate made of 90% sawdust, 9% rice bran, and 1% CaCO3 with a moisture content of approximately 65%, sterilized for 60 min at 121 °C (Stamets, 2011; Wu et al., 2017). The detailed recommendations are dependent on available raw materials, growing methods, and growing conditions. For example, Onyango et al. (2011) determined that the best A. auricula yield and quality were obtained with the use of a substrate made of maize cobs and wheat straw supplemented with wheat bran, and such combinations were recommended to wood ear mushroom growers. Devi et al. (2013) stated that mixing wheat and rice bran with paddy straw at a 3:1 ratio induced faster growth of A. polytricha in beds. Liang et al. (2019) cultivated A. polytricha on a sawdust basal substrate supplemented with different proportions of stalks of Panicum repens, Pennisetum purpureum, and Zea mays to determine the most effective substrate. The ash content of A. polytricha cultivated on a substrate containing Z. mays stalk was higher than that of the control; on the other hand, the protein content of mushrooms cultivated on a substrate containing P. repens stalk was higher than that of the control. An incubation temperature of 25–26 °C and relative humidity of 85%–90% is recommended for a spawn run for 20–25 days. The yield is approximately 1.0–1.4 kg fresh mushroom per kg dry straw in three–four flushes (Stamets, 2011). Recently, X. Y. Zhang et al. (2018) performed domestication experiments of A. villosula, and proposed conditions of spawning, manufacturing of cultural bags, spawn running, inducement of primordia, and fruiting period management to collect fruiting bodies with satisfactory performance. Similar experiments were performed by Bandara et al. (2017) with A. thailandica. In China, the major problems faced by growers of A. auricula and another species of this genus are the random labeling of strains and their introduction into different regions of identical strains under different designations (Tang et al., 2010). The precise identification and classification of commercial A. auricula cultivars is of major importance for Chinese and overseas markets.
Agaricus bisporus
Agaricus bisporus of the family Agaricaceae is grown in at least 70 countries worldwide. In the early 1990s, its global production was 1.5 million tons. Poland and the Netherlands are the largest white button mushroom producers in Europe (330 thousand tons per year) and the largest exporters of these mushrooms in the world (Kayzer, 2017). In the sixteenth century, the French were the first to cultivate white button mushrooms. This was owing to the French botanist Nicolas Marchant. The first white button mushrooms cultivated in France were considered a great rarity and were very expensive. The famous expert on cuisine, Anthelme Brillat-Savarin, valued them almost as much as truffles. This lawyer, judge, and kitchen-lover in one person in his book, Physiology of Taste, published in 1825, gave examples highlighting the taste and smell of white mushrooms, which were then served with such specialties as crayfish, capers, or the best kinds of wine and cognac (Smith, 1993). In the USA, by coincidence of manipulation with the substrate, a white button mushroom that was less aromatic but with better market worth was bred; fortunately, the original, i.e., brown mushroom, is being increasingly appreciated (Muszyńska, Kała, et al., 2017).
Agaricus bisporus, A. bitorquis, or A. subrufescens commercial cultivation is performed on compost containing lignocellulosic material as a source of carbon and nutrients for mushroom production. The compost is produced from fermented wheat straw, chicken, swine manure, and gypsum as the main raw materials (Jurak et al., 2015). Recently, many white button mushroom growers and substrate producers have been concerned about the hygiene and safety of workers, owing to their exposure to the avian influenza virus and other pathogens found in animal manure. Furthermore, composting substrate in the traditional way, in an open area, causes smell problems not only for workers but to all local society; hence, increasing attention is being devoted to finding alternative sources of nitrogen (other than those of animal origin) for mushroom substrate preparation (Altieri et al., 2009). Owing to the animal breeding industry, manure is still the cheapest and most accessible substrate. Modern white button compost producers conduct every part of production indoors, in special bunkers. With air and water recycling reducing environmental pollution, microbe inoculation to raw manure accelerates the process (Sharma et al., 2000). Phase I (fermentation) is carried out in tunnels with controlled airflow. Microbial activity increases the compost mass temperature up to 80 °C, turning carbohydrates and proteins into heat and ammonia. Phase II consists of a pasteurization process (56 °C for 5–6 hr) followed by further high-temperature fermentation (45 °C for 4–5 days). Then, the compost is spawned, and mycelium can develop in either the same or another tunnel. Phase III compost is fully colonized by mycelium, which grows in compost mass at 25 °C for 16–18 days (Kabel et al., 2017; Van Griensven & Van Roestel, 2004). The major indicators of compost quality are moisture content, C/N ratio, pH, and absence of mites, nematodes, and competitor molds (Zied et al., 2011). Sugars other than hexoses provide energy for growth and maintenance of the vegetative growth of mycelia or are metabolically converted in the mycelium and transported to the fruiting body (Patyshakuliyeva et al., 2013). The casing layer is necessary to promote fruiting; it is commonly composed of peat and limestone to correct the pH to approximately 7.8, which is optimal (Zied et al., 2011). The casing layer provides the proper conditions for the physiological change in the mycelium behavior from the vegetative to the reproductive stage. A casing layer should support microbiota-stimulating fruiting processes but must be free of mites, nematodes, and competitor molds. The high water-holding capacity and porosity of the casing layer enable water liberation without changing its structure (Martos et al., 2017). Intensively cultivated Agaricus spp. need 16–20 °C during the pinning and cropping periods. This requirement restricts white button mushroom cultivation to the temperate zone, with the best economy and quality resulting from the use of proper air-conditioning during summer months (Geösel & Győrfi, 2008).
The yield of white button mushrooms is typically expressed as kg of fresh mushrooms obtained from 1 m2 of cultivation rack. The BE% is also indicated, and for commercial profitable scale, needs to be above 80%, which corresponds to approximately 35 kg of fresh white button mushrooms from 1 m2 in three flushes (Royse & Chalupa, 2009). Under the best conditions, some commercial growers obtain a yield of more than 40 kg/m2 from three flushes. However, many white button mushroom farms are picking mushrooms from two flushes because the third flush is rarely more than 10% of the total yield, and there is a higher possibility of pest and disease that can infect other growing chambers (Sakson, 2017).
Werner & Beelman (2002) demonstrated that the addition of sodium selenite to a commercial compost in deep bags resulted in a significant increase in Se content in white button mushrooms (A. bisporus). Another issue is the high respiration rate of the fruiting bodies, leading to rapid senescence, browning, decay, and microbial infection after harvest (Song et al., 2019). The application of new packages, e.g., chitosan/zein/α-tocopherol films, can improve the antioxidant properties and maintain the quality of the mushrooms during the transport, storage, and shelf life stages (L. Zhang et al., 2020).
Ganoderma lucidum
Ganoderma lucidum from the family Ganodermataceae is one of the best-known medicinal mushrooms. It is referred to as the “mushroom of immortality” and has been used in traditional Eastern medicine for over 4,000 years. Ganoderma spp. are distributed worldwide, growing as facultative parasites or as saprobes on decaying wood (Pilotti, 2005). The mushrooms of the Ganoderma genus can form two types of fruiting bodies: a laccate fruiting body with a shiny upper surface, or a nonlaccate fruiting body with a dull upper surface (Pilotti et al., 2004). Fruiting bodies are not used as food because of their bitter taste and hardness, but have been valued for millennia as traditional medicine in China, Japan, Korea, and other Asian countries, for maintaining vivacity and longevity (Dai et al., 2009). The Chinese names, “Lingzhi,” “Chi–zhi,” or “Rui–zhi” (auspicious herb), and the Japanese names, “Reishi” (divine mushroom), “Munnertake” (10,000-year-old mushroom), or “Sachitake,” are mainly applied to G. lucidum was recorded in the first herbal manuscript “Shen Nong Ben Cao Jing” and in a poem written during the Han Dynasty 2,000 years ago (Paterson, 2006). The first Chinese Pharmacopoeia, which was written in the sixteenth century, contains a record on Lingzhi (Wachtel-Galor et al., 2005). Lingzhi is commonly found in paintings, carvings, furniture, jewelry, handicrafts, and many other artworks (Wasser & Weis, 1999). Currently, G. lucidum is not the most important species grown for a commercial purpose worldwide; Ganoderma applanatum, G. capense, G. sinense, G. tsugae, and G. neojaponicum (Chen & Chen, 2004; Tan et al., 2015) are cultivated as well. Many novel studies have been undertaken to isolate secondary metabolites of Ganoderma spp. to identify new drugs or leading compounds. Therefore, a number of bioactive constituents have been analyzed, including polysaccharides, proteins, enzymes, and polysaccharide–protein complexes (J. W. Xu et al., 2010; X. Xu et al., 2011). Ganoderma spp. extracts perform various pharmacological functions in the absence of side effects; therefore, they are widely used as the ultimate herbal substances. Ganoderma spp. have been included in the Chinese Pharmacopoeia (X. Zhou et al., 2007) and the American Herbal Pharmacopoeia and Therapeutic Compendium (Sanodiya et al., 2009).
Cultivation of Ganoderma spp. was attempted in 1937, but the first trial was performed in 1969 using spore separation. Traditional cultivation was based on the inoculation of natural logs of deciduous hardwoods without sterilization. Fruiting started after 6–24 months and continued for 5 years (Hapuarachchi et al., 2018). Natural log cultivation enables the production of fruiting bodies of superior quality that obtain the best prices in the markets of Southeast Asia. However, the growing cycle is long, and the yield is lower than that of sawdust synthetic log cultivation. It is important to conserve the forests where logs are collected, and this is a significant environmental concern (Chen & Chen, 2004). The current methods for the commercial production of Ganoderma use wood logs, short basswood segments, tree stumps, sawdust bags, and bottle procedures. Ganoderma spp. have been cultivated intensively on short wood logs and solid substrates, including grain, sawdust, tea waste, cotton seed husk, cork residues, sunflower seed hull, corn cobs, olive oil press cakes, and wheat straw (González-Matute et al., 2002; Gregori & Pohleven, 2015; Peksen & Yakupoglu, 2009; Riu et al., 1997; Ueitele et al., 2014). The most popular substrate is a mix of 75%–77% sawdust, 2% CaCO3, 5%–9% corn bran, 5%–9% oak bran, and 3.5%–4% wheat bran sterilized under standard conditions (121 °C, 1–2 hr), whereas the moisture level is maintained at 65% (Pérez-Clavijo et al., 2016). Veena and Pandey (2011) determined that the highest BE% of G. lucidum was produced with a combination of sawdust:paddy straw:rice bran of 22.5:67.5:10. The optimum temperature ranges for mycelium growth and primordial initiation are 25–30 °C and 18–25 °C, respectively. The relative humidity ranges for mycelium growth, primordial initiation, and fruiting are 60%–70%, 85%–90%, and 85%–95%, respectively. The concentration of CO2 should be above 0.1%. The light intensity should be 500–1,000 lux during primordia initiation and 3,000–50,000 lux for fruit body development (Miles & Chang, 2004; X. W. Zhou et al., 2012). Over the past few decades, the Ganoderma spp. industry has developed greatly and currently offers thousands of products to markets (Hapuarachchi et al., 2018). Some problems with Ganoderma-based products have been reported because seasonal variations, different substrate conditions, and stages of fruiting body development affect the product quality. The qualitative and quantitative differences in the chemical composition of Ganoderma spp. products are dependent on the strain, cultivation conditions, and extraction procedures (X. W. Zhou et al., 2012). Hence, it is important to develop acceptable and reproducible protocols for manufacturing processes to ensure high quality, standard, and safety of Ganoderma products (Hapuarachchi et al., 2018). Breeding of new Ganoderma strains with higher yield and resistance to diseases can increase productivity and reduce the use of chemicals for pest control. The main breeding strategies include identification, purification, and construction of gene pools, followed by conventional breeding, radiating and mutant breeding, cell and gene engineering breeding, and the evaluation of agronomic trials and biochemical characteristics (X. W. Zhou et al., 2012).
Other Cultivated Mushrooms
The enormous number of macrofungi, including a large number of species on which very little or no research has been conducted, allows us to put forward a thesis that the development of crops will continue to grow rapidly. Apart from mycorrhizal species, whose intensive cultivation under artificial conditions seems to be impossible based on current knowledge, all saprophytic species can potentially be cultivated on a massive scale. In addition to fruiting bodies, a valuable raw material for the production of dietary supplements, and potentially, medicines, is the mycelium from in vitro cultures. The mycelium obtained from bioreactors using special media has a homogeneous, constant composition of various substances with pro-health and medicinal properties, which can be easily modified by changing the conditions of cultivation (Zięba et al., 2020).
The number of new species of mushrooms that are commercially cultivated grows yearly owing to the search for new kinds of food as well as potentially new medicinal substances. Although Asian countries continue to be at the forefront of new technologies, there is a growing interest in mushrooms other than Agaricus or Pleurotus spp. in the USA and Europe. Mushrooms of the Pholiota, Flammulina, Tremella, Hypsizygus, Cordyceps, and Hericium genera are only a few types of species increasingly sought-after not only for food but also medicinal and cosmetic use (Stamets, 2011).
Calocybe indica is cultivated in polypropylene bags filled with a mix of coconut coir, kash, paddy straw, maize stalks, rice straw, sorghum stalks, sugarcane leaf, wheat bran, or waste cotton, and sterilized at 121 °C for 1 hr (Amin et al., 2010; Lakshmipathy et al., 2017).
Flammulina velutipes is produced on sawdust and rice bran contained in polypropylene bottles that are sterilized (4 hr at 95 °C and 1 hr at 121 °C), mechanically inoculated, and incubated (25 days at 20 °C). During fruiting, the temperature is lowered to 3–8 °C to obtain a better-quality yield. Mushrooms grow in a plastic collar holding the fruiting bodies in place and slow down CO2 evaporation to receive long stripes and small caps (Miles & Chang, 2004; Stamets, 2011).
Grifola frondosa cultivation is performed in polypropylene bags of 2.5 kg weight. The substrate is composed of sawdust powder, 15% rice bran, and 5% wheat bran, and 65% humidity is used. Sterilization is performed at 121 °C for 2 hr or at 110 °C for 7 hr (Mayuzumi & Mizuno, 1997).
Hericium erinaceus is commonly cultivated in polypropylene bags containing sugarcane bagasse, sawdust, cottonseed hulls, corncobs, and chopped up paddy straw mixed with rice or wheat bran, sucrose, and gypsum, and sterilized at 121 °C for 1 hr (Miles & Chang, 2004).
Hypsizygus tessulatus is cultivated in bags or bottles. The substrate is a mix of sawdust (18.2%), rice bran (8.8%), soybean shell (5.3%), corncob meal (4.7%), and CaCO3 (0.5%), and 63%–70% humidity is used. Sterilization is performed at 121 °C for 1 hr (Harada et al., 2004; Stamets, 2011).
Pholiota nameko is grown in polypropylene bags. The substrate is a mix of hardwood sawdust (90%) and corn bran, wheat bran, and dried tofu refuse (10%); a humidity of 64%–65% is used, and sterilization is performed under standard conditions of 121 °C for 1 hr (Yamanaka, 2017).
Volvariella volvácea is grown in polypropylene bags filled with substrate produced by soaking cotton wastes with a particle size of 2–3 cm in water for 24 hr and mixing with wheat bran (10%) and CaCO3 (2%), and sterilized at 121 °C for 2 hr (Philippoussis et al., 2001).
Conclusions
Commercial cultivation of medicinal and culinary mushrooms has been introduced worldwide to meet the gradually increasing demand of the food, processing, and pharmacological industries. Cultivation of edible mushrooms combines the production of protein-rich food with lignocellulosic organic waste recycling, thus meeting the pro-ecological strategies of modern agriculture. With regard to growing technologies, progress has been made in the following areas: high yield of conversion of raw ingredients to foods, limited use of pesticides for controlling pests and pathogens by using biocontrol and resistant strains, decrease in the consumption of energy by cultivating strains adapted to various climatic conditions, and greater access to technological innovations and genetic progress. The main prospects are the breeding of new strains with high yield and resistance to diseases, increasing productivity, and diminishing the use of chemicals for pest control. In addition, the improvement and development of modern engineering technologies, such as computerized control of environmental parameters, utilization of new substrates, methods for their sterilization, and spawn preparation, can increase the productivity. All these aspects will be crucial in the production of mushrooms with better appearance, texture, nutritional qualities, and medicinal value. Good laboratory, agriculture, manufacturing, production, and clinical practices are essential to achieve quality mushroom products, which can be used as new mineral sources in dietary supplements or as value-added ingredients for the formulation of functional foods or nutraceuticals.
Handling Editor
Wojciech Pusz; Wrocław University of Environmental and Life Sciences, Poland; https://orcid.org/0000-0003-1531-2739