. Introduction
Light is one of the most dynamic environmental factors that directly affect plant performance; therefore, it is important to understand how plants acclimate to light changes (Ruban, 2015). Plants can adjust their photosynthetic apparatus to a wide spectrum of daily and seasonal fluctuations in light conditions (Vialet-Chabrand et al., 2017). Acclimation to different light intensities and qualities is manifested by changes in the quantum yield of photosynthesis and the relative levels of the thylakoid components, including photosystems I and II (PSI and II; Bailey et al., 2001; Kono & Terashima, 2014, 2016). C4 plants have evolved adaptation mechanisms to cope with high irradiance in their natural habitats to optimize the utilization of the absorbed light energy and minimize photodamage (Osborne & Sack, 2012). Thus, low light (LL) levels favor the accumulation of the C3 fixation enzyme Rubisco, whereas high light levels lead to the accumulation of both Rubisco and the C4 fixation enzyme phosphoenolpyruvate carboxylase (PEPC) (Bassi & Passera, 1982; Langdale et al., 1988; Nelson & Langdale, 1989). Both biomass production and CO2 fixation increase at a faster rate with higher light intensity and CO2 concentration in C4 plants than in C3 plants, which reflects the more efficient use of light and CO2 in C4 plants (Wang et al., 2012). The photosynthetic rate and biomass of different C4 subtypes depend on the environmental conditions, including the water and nitrogen supply (Buchman et al., 1996). Although the basic features of C4 photosynthesis are well understood, the quantitative significance of the elements that are responsible for high photosynthetic efficiency is not well defined for C4 photosynthesis.
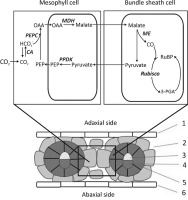
Most C4 plants contain two types of photosynthetic cells, the mesophyll (M) and bundle sheath (BS) cells, which differ structurally and functionally, each with a distinct chloroplast type.
In C4 photosynthesis, Rubisco is localized only in the interior cell layer, surrounding the vascular tissues known as the BS tissues (Kranz anatomy; Hatch, 2002; von Caemmerer & Furbank, 2003). In C4 plants, there are two M cells between neighboring vascular bundles, and the BS cells in C4 plants are large and contain more chloroplasts than the M cells (Figure 1). There are three C4 subgroups based on the differences in the decarboxylation mechanisms (Hatch, 1999): NADP-malic enzyme (NADP-ME), NAD-malic enzyme (NAD-ME), and phosphoenolpyruvate carboxykinase (PEPCK). CO2 enters the M cell cytoplasm where it is first converted to bicarbonate ions by carbonic anhydrase and then fixed by PEPC localized in the M cells, which binds the bicarbonate to phosphoenolpyruvate (PEP) to form a four-carbon organic acid, oxaloacetic acid (OAA; Figure 1). Then, OAA is modified to malate or amino acid aspartate and is transported to the BS tissue where Rubisco is localized. In the BS cells, the four-carbon acid is decarboxylated to release CO2 and form a three-carbon product, pyruvate, or alanine. Next, the three-carbon organic acids resulting from the decarboxylation reactions are returned to the M cells, where they are used to regenerate the CO2 acceptor pool. Lastly, the released CO2 accumulates at a high concentration in the BS chloroplasts where it is refixed by Rubisco, and such highly concentrated CO2 largely inhibits the binding of oxygen by Rubisco; thus, photorespiration is very low (von Caemmerer & Furbank, 1999).
Figure 1
Leaf structure and C4 metabolic pathways of maize [NADP-malic enzyme (NADP-ME) type]. Enzymes: carbonic anhydrase (CA), phosphoenolpyruvate carboxylase (PEPC), malate dehydrogenase (MDH), NADP-ME and pyruvate, and orthophosphate dikinase (PPDK). Labels: 1, 6 – epidermis; 2 – mesophyll cell; 3 – xylem; 4 – phloem; and 5 – bundle sheath cell.
Accumulating evidence indicates that many C4 plants use a combination of organic acids and decarboxylases during CO2 fixation and that the C4-type categories are not rigid (Bräutigam et al., 2014; Furbank, 2011). The ability to transfer multiple organic acid species and utilize different decarboxylases has been suggested to give C4 plants an advantage in variable and stressful environments by facilitating an energy balance between the two cell types involved in CO2 assimilation (Ludwig, 2016). Meister et al. (1996) suggested that malate decreases PSII activity in BS cells because the replacement of malate by aspartate as a source of CO2 in the BS cells of NADP-ME species decreases the reducing equivalents transferred to these cells, and the role of aspartate is to increase PSII activity in the BS cells to compensate. However, little information is available on how combination pathways may enable C4 plants to mitigate the effects of fluctuating environmental factors or stress. The presence of alternative decarboxylation pathways, with differing contributions to M and BS cell energy demands, is important for maintaining cell-type energy balance and high CO2 assimilation rates in the leaves under different light conditions (Bellasio & Griffiths, 2014). Each of the C4 subtypes is characterized by specialized leaf anatomy, and there is a correlation between anatomy, ultrastructure, and the decarboxylation mechanism of BS (Kranz) chloroplasts. The NADP-ME species have chloroplasts that are agranal and located toward the outer part of the Kranz cells. Also, the PEPCK species have granal chloroplasts located near the centrifugal position, whereas the NAD-ME species have chloroplasts in the centripetal position with grana (Rao & Dixon, 2016). In addition, in some C4 species, there is chloroplast size dimorphism, with the BS cell chloroplasts being larger than the M chloroplasts (Laetsch, 1971; Voznesenskaya et al., 2006). In NAD-ME and PEP-CK species, the chloroplasts of the BS may be photosynthetically very similar to those of the M; however, this is yet to be fully investigated. The concentric anatomy of C4 leaves allows light to reach the M cells before the deeper BS cells (Long et al., 1989) and modifies the light-harvesting process. In a previous study (Zienkiewicz et al., 2015) it was shown that the response of C4 plants to light is species-dependent because chloroplasts are stimulated differently; this is probably due to differences in light penetration across the leaves and the redox status of the chloroplasts, which influences the C and N metabolisms.
Light signals are interpreted by plants via the transduction cascade and enzymatic activity. The response to light involves the activation of gene expression and posttranslational modification of proteins; light also activates phosphorylation and redox cascades. Transcripts involved in protein synthesis, folding, and assembly are more abundant in M cells than in BS cells in NADP-ME plants. The differentiation of transcriptional and post-transcriptional regulatory mechanisms in both NADP-ME type cells might be associated with the unequal distribution of metabolites within the cells (Rao et al., 2016). For instance, the messenger RNA (mRNA) of light-harvesting complex II (LHCII) and LHCII gene translation product(s) accumulate at a lower level in BS cells than in M cells (Vainstein et al., 1989). This could be because of transcriptional/post-transcriptional control and a less efficient transport system for LHCII in BS cells than in M cells. Covshoff et al. (2008) showed that in C4 plants, the relative levels of gene transcripts do not correlate with the corresponding protein levels, suggesting the involvement of transcriptional/translational regulation during C4 differentiation. For acclimation to light intensity, the M and BS chloroplasts of Zea mays use different mechanisms of adjustment and optimization of their functions, depending on the irradiance conditions prevailing during their growth. Light acclimation to high light in maize is a tightly coordinated by adjustment of the light reaction components/activity to balance light utilization in both the M and BS chloroplasts (Drożak & Romanowska, 2006).
. Structure and Function of Photosystem II in Agranal Maize Bundle Sheath Chloroplasts
The literature agrees with the existence of an active PSI in NADP-ME plants in both cell types; however, there is disagreement about the existence of PSII activity and the presence of some PSII proteins in maize BS cells (Edwards & Huber, 1981). It has been suggested that agranal chloroplasts have less PSII activity than granal M chloroplasts, associated with reduced amounts of some proteins and mRNAs for PSII components (Schuster et al., 1985). Additionally, it is argued that the agranal BS chloroplasts of maize are typical of the genus and do not appear to be influenced by irradiance (Downton & Pyliotis, 1971) and that the PSII activity of BS thylakoids is very low, if at all detectable (Ghirardi & Melis, 1983; Leegood et al., 1983; Usuda et al., 1975), and they have low water oxidation capacity (Edwards & Walker, 1983; Ivanov et al., 2005; Mayne et al., 1974). For instance, Bazzaz and Govindjee (1973) showed using fluorescence characteristics that total chlorophyll a fluorescence in the BS chloroplasts of maize is 40% lower than that in M chloroplasts, and electron transport is present in both types of chloroplasts. There have also been other reports on functional PSII complexes in the BS chloroplasts of maize, which claim that it is up to 50% of the whole-chain electron-transport capacity seen in the thylakoids of C3 plants (Hardt & Kok, 1978; Walker & Izawa, 1979). Furthermore, Pfündel et al. (1996) showed using fluorescence measurements of pure M and BS thylakoids using flow cytometry that the excitation energy of the PSII complex in BS chloroplasts contributes significantly to the functional antenna of PSI despite their relatively low concentrations. These data agree with the suggestion of Bassi et al. (1995) that the PSII LHCII antenna functions as an antenna for PSI in BS chloroplasts. Nevertheless, it was also suggested that BS thylakoids contain only traces of LHCII and lack polypeptides of the oxygen-evolving complex (OEC) and ferredoxin NADP reductase. These discrepancies may occur because of differences in tissue age and different light intensities during growth and could be due to the cross-contamination of BS with M chloroplasts during chloroplast isolation.
In C4 plants (NADP-ME type), where chloroplasts are differentiated into M (granal) and BS (agranal) types (Figure 2A), BS thylakoids generally exhibit only PS I activity and contain traces of PSII. As conflicting data on PSII activity in BS chloroplasts have been reported, the degree of PSII deficiency in BS chloroplasts of NADP-ME species has always been a matter of debate (Edwards & Huber, 1981). Examples that are contrasting to BS chloroplasts in maize could include other NADP-ME species, such as Echinochloa crus-galli and Digitaria sanguinalis, representing the scope of variation in the abundance of grana in BS chloroplasts (Ueno et al., 2005). It has also been demonstrated that the level of PSII in maize is regulated by the synthesis of key core components (Meierhoff & Westhoff, 1993), and it has been postulated that the PSII content of BS chloroplasts may be proportional to the amount of aspartate that is transported from the M to BS cells (Chapman & Hatch, 1981; Meister et al., 1996). Furthermore, studies on NADP-ME subtype monocots found that M chloroplasts had a PSII activity that was approximately tenfold higher than that in BS chloroplasts when compared on a chlorophyll basis (Ketchner & Sayre, 1992). Undoubtedly, great variation in the observed PSII composition and activities in the BS chloroplasts of maize (Table 1) could either be due to different experimental assays or the cross-contamination of one cell type as a result of the harsh procedure that is necessary for chloroplast isolation from the robust maize BS tissue.
Figure 2
(A) Schematic of maize (NADP-malic enzyme C4 type) mesophyll and bundle sheath chloroplasts. (B) Model of State 1 to State 2 transitions in maize chloroplasts, where mesophyll chloroplasts have typical State 1 to State 2 transition; whereas bundle sheath chloroplasts have permanent State 2, where light-harvesting complex II antennas are bound to photosystem I (Rogowski et al., 2018).
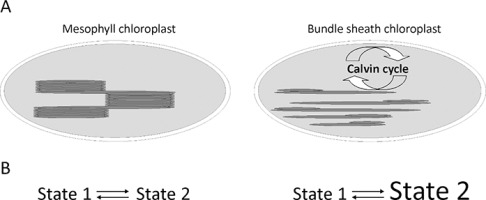
Table 1
Summary of the properties of PSII in BS chloroplasts in maize.
| Description | Reference |
|---|---|
| PSII monomer | Bassi et al., 1995 |
| PSII dimer | Romanowska, Kargul, et al., 2008 |
| Lack of smallest proteins of LHCII | Vainstein et al., 1989 |
| High amount of LHCII with respect to D1 | Bassi et al., 1995; Drożak & Romanowska, 2006 |
| Absence of LHCII and PSII | Broglie et al., 1984 |
| Presence of OEC and LHCII in low amount | Sheen & Bogorad, 1987 |
| Lack of OEC | Lu & Stemler, 2002; Schuster et al., 1985 |
| Traces of LHCII | Schuster et al., 1985 |
| Lack of 33 kDa protein of OEC | Bassi et al., 1995 |
| Linear electron transport not occurs | Bassi et al., 1995 |
| Linear electron transport is present | Bazzaz & Govindjee, 1973 |
| Deficiency of PSII | Polya & Osmond, 1972; Usuda et al., 1975 |
| Lack of PSII activity | Ku et al., 1974; Schuster et al., 1985; Woo et al., 1970 |
| Lack of O2 evolution | Leegood et al., 1983 |
| Capacity for O2 evolution | Chapman et al., 1980; Edwards & Walker, 1983; Ivanov et al., 2005; Mayne et al., 1974; Woo et al., 1970 |
Consequently, we attempted to re-evaluate the photochemical activities of chloroplasts in BS cells by optimizing the conditions of the isolation procedure. Two different methods for the isolation of BS chloroplasts (mechanical and enzymatic) were compared to address the question of whether PSII is inactivated during the differentiation of maize leaves or the isolation procedure. Romanowska et al. (2006) provided evidence that all the protein components of the PSII complex are present in maize BS chloroplasts and net O2 evolution was not detected, but the electrons were transferred from water through PSII using DCPIP. These results suggest that PSII acts as a poising agent for PSI and it is required for cyclic electron flow in BS chloroplasts. Furthermore, the organization of PSII in the BS thylakoids in maize was investigated using blue native/sodium dodecyl sulfate polyacrylamide gel electrophoresis and single particle analysis, and for the first time, it was shown that PSII in BS chloroplasts exists in a dimeric form and forms LHCII-PSII supercomplexes (Romanowska, Kargul, et al., 2008). Also, earlier data suggested that residual PSII activity in BS chloroplasts may supply electrons to control cyclic electron flow around PSI and prevent PSI over-oxidation, which is essential for CO2 fixation in BS cells, and hence may optimize adenosine triphosphate (ATP) production within this compartment. This supports the view that the photosynthetic apparatus of the maize BS chloroplasts has limited noncyclic phosphorylation. Thus, PSII in BS agranal chloroplasts is not equivalent to PSII in the stromal membranes of C3 plants.
. Changes in the Activity and Structure of Mesophyll and Bundle Sheath Chloroplasts in Response to Light Intensity
Romanowska et al. (2017) studied whether the chloroplasts of NADP-ME species possess the same mechanisms capable of inducing similar responses to high light conditions, thereby maintaining high photosynthetic efficiency. For the first time, they found that different NADP-ME species, Zea mays, Echinochloa crus-galli, and Digitaria sanguinalis, cultivated under the same light conditions differed in their photosynthetic and respiratory responses to high light treatment (Romanowska et al., 2017). These differences might be due to the imbalance in the excitation energy between the photosystems present in the M and BS cells and the differences in the penetration of light inside the leaves. It was found that the non-radiative dissipation of energy in the studied plants was not dependent on carotenoid content, and the activity of the antioxidant enzymes and the malondialdehyde and H2O2 levels were similar. This suggests that other photoprotective mechanism(s) might have been involved in NADP-ME species under high light stress (Romanowska et al., 2017). Among the investigated species, E. crus-galli was best adapted to the high light treatment. The high resistance of the photosynthetic apparatus of E. crus-galli toward photoinhibitory light was accompanied by elevated levels of phosphorylation of the PSII protein subunits, photochemical quenching, organization of the PSI and PSII complexes, integrity of the thylakoid membranes, and an elevated ATP/adenosine diphosphate (ADP) ratio.
The effects of environmental factors (level of irradiance, temperature, and heavy metals) on photosynthesis and the abundance of components of the thylakoid complexes and antenna systems in C4 plant subtypes are not well known. There is also limited knowledge on the ability of C4 plants to modulate the photosynthetic apparatus depending on the plant species, growth conditions, and intensity of the environmental stresses. The energy requirements of the cell types of C4 species vary because of the differences in the decarboxylation mechanism and the respective C4 cycles. These differences in the energy requirements are reflected in the differences in the chlorophyll a/b ratio and the distribution of the photochemical activities between cells. Generally, NADP-ME species have relatively high activities of non-cyclic electron transport in M chloroplasts and cyclic electron flow in BS cells (Munekage & Taniguchi, 2016). Whereas in NAD-ME and PEPCK species, the main photochemical activity is localized in BS cells. The ATP that is required to convert pyruvate to PEP can be provided by cyclic, noncyclic, or pseudocyclic electron transport (Bellasio & Griffiths, 2014). Cyclic phosphorylation in NADP-ME M chloroplasts occurs at a higher light saturation than noncyclic and pseudocyclic photophosphorylation (Edwards et al., 1979). Thus, qualitative changes in ATP may be generated by different types of electron flow (Takabayashi et al., 2005). At high light intensities, increased cyclic photophosphorylation may contribute to the C4 pathway (Romanowska, Drożak, et al., 2008). Nevertheless, there have been relatively few studies on photophosphorylation in the chloroplasts of PEPCK and NAD-ME C4 plants, and no distinction was made between M and BS origin (Edwards & Walker, 1983) or high cyclic phosphorylation capacities that were observed in the BS chloroplasts of NADP-ME plants (Chapman et al., 1980; Leegood et al., 1981).
Earlier results demonstrated that M and BS chloroplasts of the three C4 subgroups (NADP-ME, NAD-ME, and PEPCK) that grew under the same light conditions differed significantly with respect to organization and activity (Romanowska & Drożak, 2006). For instance, Z. mays (NADP-ME) leaves that were acclimated to different light conditions showed changes in the LHCII complexes in both types of chloroplasts, whereas in NAD-ME and PEPCK plants, although some changes occurred at the chloroplast organization level, the abundance of the light-harvesting complex proteins was similarly affected (Romanowska, Powikrowska, et al., 2008). In all the examined species, the intensity of the light caused changes in the plastoquinone (PQ) levels, which suggests that the PQ pool and its changes in the redox state are associated with different acclimation responses among the C4 subtypes. An additional candidate is the ATP content in both the M and BS chloroplasts. However, it is unclear why such responses to light are different in the C4 subtypes (differing in the amounts of the thylakoid components and their activity and/or the chloroplast ultrastructure). This is probably related to a photoprotective strategy for the better utilization of absorbed light in the different subtypes. However, the differences in photosynthetic activity probably reflect the rearrangement of the membrane complexes. Since the PQ pool differs significantly in M and BS chloroplasts under the same light conditions and between subtypes, this may suggest that the acclimation plasticity of C4 plants with respect to light intensity and quality is differently regulated by phosphorylation, which affects the energy distribution between photosystems.
It was shown that photosystem II in maize M thylakoids contained (on average) 1.5 ± 0.1 or 2.3 ± 0.2 phosphoryl groups in plants grown under either low or high light, while in BS membranes there were 0.25 ± 0.1 or 0.7 ± 0.2 phosphoryl groups, respectively (Fristedt et al., 2012). This strongly suggests that the structure of the thylakoid membrane governs both the phosphorylation and turnover of photosystem II. For all the C4 subtypes, the light intensity had a strong effect on electron transport through PSI and PSII in both the BS and M chloroplasts. However, the light intensity did not affect the level of D1 protein (Pokorska & Romanowska, 2007), but high light increased the accumulation of CF1 and cytochrome b6f proteins in the M and BS chloroplasts of all tested C4 plants (Romanowska & Drożak, 2006; Romanowska, Kargul, et al., 2008; Romanowska, Powikrowska, et al., 2008). Furthermore, in an earlier study on the effects of irradiance on C4 chloroplasts, a significant increase in ATP synthase activity in the M and BS chloroplasts was observed in response to high light. The results showed that the two CF1α isoforms coexist in the chloroplasts; the CF1α ′ isoform is more abundant in high light-grown plants than in low light-grown plants (Romanowska, Powikrowska, et al., 2008). It was suggested that in the M and BS chloroplasts of C4 plants a mechanism(s) regulates the ATPase composition in response to light irradiance. Thus, accumulation of the α ′ isoform may have a protective role under high light stress against over-protonation of the thylakoid lumen and photooxidative damage to PSII. The data also indicated that ATP production in C4 plants is regulated by two separate mechanisms: one under normal growth conditions and the other when plants are exposed to stressful environments. Therefore, the metabolic separation of M and BS cells plays an important role in the acclimation process because it enables the regulation of the ATP/ADP ratio in both types of chloroplasts.
. Light Regulation of Photoinhibition, Photosystem II Turnover, the Phosphorylation of Proteins, and the Composition of the Thylakoid Membranes of the Mesophyll and Bundle Sheath Chloroplasts
The BS chloroplasts in the NADP-ME subtype are agranal (cyclic electron transport dominate) with structures similar to that of the stroma lamellae of C3 chloroplasts. Whereas the BS and M chloroplasts in both the NAD-ME and PEPCK subtypes are granal (with cyclic and noncyclic electron transport); however, it is unknown whether the structural and functional heterogeneities in PSI and PSII exist in these subtypes. Some data concerning the NADP-ME subtype are incompatible, presumably because of environmental factors, especially light intensity and temperature, which cause substantial changes in the aggregation of the antenna systems, pigment content, and photosystem efficiency. Dynamic and reversible changes in the composition of the thylakoid membranes have great importance in sustaining the photosynthetic and respiratory activities of plants under variable and stressful conditions. Also, the efficiency of metabolite exchange between the BS and M cells may be important. The ATP and/or NADPH produced in the light reactions of both types of C4 cells can regulate not only photosynthetic metabolism but also mitochondrial respiration. However, little is known about the precise mechanisms of acclimation to light in both types of chloroplasts. Nevertheless, it was established that M and BS chloroplasts of various C4 plant species differ with respect to the photosynthetic membrane (thylakoid) organization and activity, even when exposed to the same light conditions (refs from Romanowska’s lab). Recent work points to dynamic changes in the redox balance of the PQ pool (the electron carrier that mediates electron transfer between PSII and PSI) and the ATP level as the two key strategies that have evolved in both types of chloroplasts in the various C4 plant species for acclimation to light. Nevertheless, little is known about how these processes regulate the dynamics of the adaptive responses to light in various C4 plants, which are manifested by changes in the macroorganization of photosynthetic membranes, such as the remodeling of the grana stacks and their key components, light-harvesting complex I (LHCI) and LHCII, and changes in the stoichiometry of the photosynthetic complexes.
Plants grown under stable environmental conditions develop mechanism(s) that are responsible for the optimal efficiency of photosynthesis, and the redox signal derived from photosynthetic electron transport plays an important regulatory role by modulating the expression of genes that encode photosynthetic components (Allen, 2002, 2004; Walters, 2005). It is also possible that under constant conditions, the redox changes are too small to be measured, but plants still respond to these changes. When plants are suddenly subjected to stress conditions (e.g., low light → high light), other acclimation mechanisms may be activated, including metabolic signals, alteration in the ATP/ADP ratio, and turnover of the D1 protein from the reaction center of PSII. The rapid turnover of the D1 protein is an example of the plasticity of the photosynthetic apparatus and prevents the inhibition of photosynthesis in plants under variable environmental conditions, especially variations in light intensity and low temperatures (Allakhverdiev & Murata, 2004; Andersson & Aro, 2001). The rate of D1 protein degradation is regulated by its phosphorylation and magnesium and ATP levels (Rintamäki, Kettunen, & Aro, 1996). It has been shown that D1 can only be digested in its dephosphorylated form (Ebbert & Godde, 1996). Additionally, PSII core proteins, including D1, are phosphorylated in grana thylakoids, whereas their dephosphorylation and degradation occur in non-appressed membranes (Rintamäki, Salo, et al., 1996), where most thylakoid proteolytic enzymes are localized (Haussühl et al., 2001; Itzhaki et al., 1998). Illumination leads to reversible phosphorylation of photosystem II proteins and phosphorylation of LHCII increases the transfer of energy to PSI (state transitions). Also, LHCII kinase is activated by the over-excitation of PSII. Therefore, reversible phosphorylation regulates the relative rates of cyclic and noncyclic electron transport and coordinates the rate of ATP synthesis. The two photosystems did not have identical light-absorption spectra and spectral light-use efficiencies. These differences arise from the differences in the pigments and the binding of the pigments in the two photosystems. Therefore, changes in the spectra have great potential to disrupt the balanced activities of PSI and PSII, which are required for optimal linear electron transport (Laisk et al., 2014). Thus, state transitions represent a fast excitation-balancing mechanism that operates in minutes in response to imbalances in electron transport through PSI and PSII (Allen et al., 1981).
Hence, chloroplasts of NADP-ME C4 species with different amounts of grana systems in BS chloroplasts are a good model for investigating the mechanism(s) that are responsible for the optimal production of energy and redox equivalents for CO2 assimilation. High ATP concentrations in chloroplasts increase protein phosphorylation while simultaneously inhibiting phosphatases (Rintamäki, Kettunen, & Aro, 1996). In contrast, the ATP level also appears to affect the activity of proteases (Lindahl et al., 2000). PSII in the BS membranes of maize contains all the polypeptides involved in electron transport and oxygen evolution, but they are with very low activity (Romanowska et al., 2006). Pokorska and Romanowska (2007) demonstrated that the D1 degradation cycle is very efficient in BS maize thylakoids, confirming that PSII plays an important role in these membranes. The relative amount of Deg1 proteases in Z. mays BS chloroplasts is significantly higher than that in M chloroplasts, despite the lower content of PSII (20%) in the BS than in the M chloroplasts (Pokorska et al., 2009). Furthermore, protease Deg1-mediated degradation of not only the photosystem II core proteins D1/D2 but also the minor LHCII proteins CP26, CP29, and PSII-associated PsbS protein were found in both M and BS chloroplasts (Zienkiewicz et al., 2012). It has been postulated that PsbS, CP26, and CP29 are the main players in the dissipation of excess excitation energy and adaptation to changing light conditions during state transitions (Bonente et al., 2008; Kargul & Barber, 2008). This indicates the possible involvement of Deg1 in the regulation of these photoprotective mechanisms by cleavage of the photodamaged PSII core and antenna subunits in the C4 plant Z. mays. Therefore, BS chloroplasts of the NADP-ME species of C4 plants with different amounts of grana (10%–30% as compared to that in M chloroplasts; Hatch, 1987) provide an appropriate model to study the interrelations between the ultrastructure, electron transport, and turnover processes.
The extent of acclimation varies between C4 species per the metabolic differences among chloroplasts and differences in energy demand (Edwards et al., 2001). It was found that in BS chloroplasts of a PEPCK plant (Panicum maximum), where the demand for ATP is higher than that in NAD-ME plants (Panicum miliaceum), the accumulation of ATP synthase isoforms was stimulated by high light intensity (Romanowska, Powikrowska, et al., 2008). This is in addition to the observation that in maize (NADP-ME subtype), high light increased the level of the CF11α isoform. This may indicate that the same signal operates at both low and high light intensities. Therefore, the extent of acclimation would not simply depend on the photon flux density but rather depend on both the protein content and intensity of electron transport. The acclimation to the light intensity that was observed for the CF1α isoforms in both the granal and agranal chloroplasts suggests that this kind of response to light is universal. The CF1α isoforms, regardless of their physiological significance, might be a general feature of chloroplast ATP synthase complexes in many other plant species. In the BS chloroplasts of NADP-ME species, PSI activity is very high, which supports the view that the photosynthetic apparatus of BS chloroplasts generates ATP through a PSI-dependent cyclic pathway of electron transport (Ivanov et al., 2007).
Therefore, the following question arises: what is the function of PSII in BS agranal chloroplasts? Pfündel et al. (1996) showed that the excitation energy of PSII was efficiently transferred to PSI in the BS chloroplasts of many NADP-ME species despite the relatively low concentrations of PSII, but they did not demonstrate that the light energy was used for ATP synthesis. Earlier results suggested that differences in ATP synthase activity may play a role in the regulation of photosynthetic energy conservation to allow for flexibility in the stoichiometry of the ATP/NADPH ratio (Romanowska & Drożak, 2006). Since PSII in the maize BS chloroplasts exists in the dimeric form and forms LHCII-PSII supercomplexes (Romanowska, Drożak, et al., 2008), this suggests that residual PSII may supply electrons to poise cyclic electron flow around PSI and prevent PSI over-oxidation, which is essential for CO2 fixation in BS cells, and hence, may optimize ATP production within this compartment (Romanowska, Kargul, et al., 2008). Thus, the role of PSII would be complementary to that of NADH-PQ reductase, which is enriched in the BS chloroplasts (Darie et al., 2006) and injects electrons into the PQ pool depending on the redox status of the stroma.
It was hypothesized that in BS chloroplasts, some of the LHCII antennas can be permanently bound to PSI and that cyclic electron transport is highly stimulated by ATP synthesis. It is unknown how the redox state of PQ influences kinase activity and what is the role of the cytochrome b6f complex in the BS. Thus, in C4 plants, short-term acclimation to changes in light intensity and quality are likely to be mediated by redox-dependent kinases, which are involved in the signaling cascades that ultimately lead to the phosphorylation and structural rearrangement of the light-harvesting antennas that are associated with both photosystems in the process of state transitions (Kargul & Barber, 2008). Phosphorylation of the key proteins in the photosynthetic membrane regulates the profound reorganization of the electron transfer chain and remodeling of the thylakoid membranes (Allen, 1992; Bennett, 1977). It is a mechanism for reconfiguring the photosynthetic light-harvesting apparatus in response to changing light conditions through phosphorylation of LHCII (Tikkanen et al., 2010; Wollman, 2001). This is achieved by the migration of a part of the LHCII antenna between PSI and PSII (Pesaresi et al., 2011). In plants, state transitions are typically induced by changing the spectral quality of the light: far-red (FR) light that favors PSI is used to promote State 1, while orange or blue wavelengths that favor PSII are used to induce State 2. Plants under low light intensities are usually in State 2 when some of the LHCII antennas are bound to PSI. Thus, “state transitions” act as a mechanism for balancing the excitation of the two photosystems under changing light regimes. Furthermore, earlier results showed that the short-term action of FR light has a strong effect on LHCII protein phosphorylation in BS chloroplasts, and stimulates PSI activity in these chloroplasts (Zienkiewicz et al., 2015). Rogowski et al. (2018) provided novel insights into the connections between the membrane structures of M and BS chloroplasts, protein phosphorylation, and photosystem function. In M chloroplasts, FR light induced dephosphorylation of LHCII and detachment of the antenna from PSI. In the BS thylakoids, FR light initiated the dephosphorylation of free and aggregated LHCII antenna. The dephosphorylated antenna can bind to PSII, which strongly increases its fluorescence, while part of the LHCII pool remains associated with PSI. Thus, aggregates of LHCII tended to be resolubilized in FR light, which allowed LHCII to connect to PSII without changes in the pool of PSI-LHCI-LHCII. These results indicate that M chloroplasts have “state transitions,” as is known to exist in C3 plants, whereas BS chloroplasts exhibit a uniquely permanent State 2, where LHCII is associated with PSI (Figure 2B); this is independent of the light quality. The permanent presence of State 2 in the maize BS chloroplasts may be aimed at maximal functioning of cyclic electronic flow around PSI for ATP production; whereas changes in the PSII antenna may change the intensity of linear electron flow for PSI protection. This indicates that the standard “state transitions” model must be revised. Such regulation is likely necessary for the efficient use of light in C4 plants.
Furthermore, for the first time, research was conducted on the organization of complexes in the stroma lamellae and margin regions of maize M chloroplasts, where different types of complexes are expected to occur (Urban et al., 2020). Grieco et al. (2015) suggested that in addition to the movement of LHCII, entire photosystems may also be relocated during “state transitions.” This allows for direct energy transfer from PSII to PSI under light, which preferentially excites PSII. The movement of PSII-LHCII toward the margins allows for the mixing of the photosystems and an energetic linkage between them. It was found that in these membranes, PSI may simultaneously exist in two forms: the most abundant type being composed of PSI-LHCI-PSII-LHCII megacomplexes, and the other type being composed of PSI-LHCI-LHCII supercomplexes (Urban et al., 2020). These complexes were formed under both low and high light growth conditions but at different concentrations. Changes in the levels of the megacomplexes are controlled by the light intensity during growth and actual light quality. The results suggest a different function of super- and megacomplexes organization than the classic “state transitions” model, which assumes that the movement of LHCII trimers in the thylakoid membranes is a mechanism for balancing light absorption between the two photosystems under light stress. Light-dependent modifications control both the docking and disconnecting of PSI and PSII proteins; thus, they can balance energy distribution between the two photosystems, can participate in the repair of the photosystems, and, as can be seen from the effects of the growth light, can participate in energy dissipation. Therefore, the behavior of the described complexes does not seem to be well explained by the “state transitions” model, rather, the role of these complexes in excitation quenching for PSI and turnover for PSII is indicated. Schwarz et al. (2018) suggested that in C3 and C4 (NAD-ME type) plants, PSI-LHCII megacomplex formation requires thylakoid stacking; depends on the growth light intensity, leaf age, and PSII core phosphorylation; and is correlated with changes in the PSI/PSII ratios. They proposed that the migration of LHCII antennas protects PSI against excessive stimulation and regulates PSII turnover and, thus, repair the cycle.
Although PSII is extremely sensitive to variable environmental factors, it shows great potential for adaptation to diverse environmental conditions. The molecular mechanisms of PSII quantum efficiency optimization and defense against photodamage provide the basis for a better understanding of the plasticity of the photosynthetic apparatus. Nevertheless, little is known about how these processes regulate the dynamic adaptive responses to light in various C4 plants, which are manifested in changes in the macroorganization of the photosynthetic membranes, such as changes in the grana stacks and their key components, the LHCI/II, and the stoichiometry of the photosynthetic complexes. This knowledge will provide insights into the significance of these systems in C4 plant physiology and ecology, contribute to attempts to increase C4 crop yield and ensure global food security, predict the effects of different climate change scenarios on natural and agricultural C4 species-rich environments, and assist with designing future strategies in plant biotechnology.
. The Influence of Light Penetration Into the Leaves on the Metabolite Distribution and Structure and Function of Mesophyll and Bundle Sheath Cells
C4 photosynthesis is related to the intensive exchange of intermediates between the photosynthetic cells. The diffusion of intermediates in C4 photosynthesis subtypes is driven by an intermediate concentration gradient (Leegood, 2000) and the plasmodesmata at the interface of M and BS cells are particularly numerous, possibly enabling efficient metabolite diffusion (Botha & Evert, 1988; Evert et al., 1977). This hypothesis is supported by a positive correlation between the photosynthetic rate and the plasmodesmata number in a few grass species originating from South Africa with different photosynthesis subtypes (Botha, 1992). This relationship has not been studied in other species, and the short-distance transport of photosynthates has not been studied directly. Nevertheless, short-distance transport studies have been conducted on maize (Danila et al., 2016; Sowiński, 1998; Sowiński et al., 2007).
When studying the compartmentation of the enzymes that are involved in the photosynthetic carbon metabolism in the different chloroplast-containing tissues of various C4 species, the partitioning of carbon into carbohydrates is unclear. It has been proposed that sucrose is predominantly synthesized in the M cells, whereas starch is normally synthesized in the BS cells. However, the enzymes involved in their biosynthesis are found in both cell types (Leegood & Walker, 1999). To determine the distribution of the metabolites between the M and BS cells during C4 photosynthesis, fluorescence techniques were used and short-distance transport was measured. Additionally, in the NAD-ME and PEPCK subtypes, the mitochondria of the BS cells are also involved in photosynthesis, since acid decarboxylation by mitochondria-located NAD-specific malic enzymes provides ATP for the cytosol-located PEPCK (in PEPCK species). The importance of mitochondrial activity for carbon and nitrogen metabolism in C3 plants has been well documented (Atkin et al., 2000; Krömer, 1995), however, little is known about their activity under light and darkness in the leaves of C4 plants. Studies (Rogowski et al., 2019) have shown that the respiration rate of C4 leaves, similar to that in C3 leaves, also increases following illumination/photosynthesis. However, this increased respiration, termed light-enhanced dark respiration, was dependent not only on the amount of CO2 fixed but also on the light reactions. This provides evidence for a link between photochemical activity and mitochondrial respiration.
Notably, in maize, the rate of photosynthesis is dependent on the light intensity during measurement (actual light intensity) and not on the growth light intensity. Coordination of both the light and dark reactions of photosynthesis results in a high efficiency of this process under changing light conditions. For acclimation to light intensity, M and BS chloroplasts use different mechanisms of adjustment and optimization of their functions, depending on the irradiance conditions prevailing during growth, and these mechanisms are associated with different light penetration across the leaf. Zienkiewicz et al. (2015) showed that light quality has various effects on photochemistry and protein phosphorylation in Z. mays thylakoids, owing to the different degrees of light penetration across the leaves and the redox status in the chloroplasts. These acclimation changes that are induced by light quality are important in the regulation of chloroplast membrane flexibility and function. Thus, the consequences of light gradients through a leaf have been identified in the chloroplast ultrastructure (Terashima et al., 1986) and photosynthetic function (Evans & Vogelmann, 2003), showing that differential acclimation occurs for individual cells or chloroplasts according to their position within a leaf. Therefore, differences in the light quality penetration into a leaf are likely to have profound impacts on C4 photosynthesis because the C4 photosynthetic pathways require metabolic coordination of the M C4 and BS C3 cycles. Additionally, it is known that the rate of photosynthesis is reduced under blue light compared to red or green light (Evans & Vogelmann, 2003; Loreto et al., 2009) and this is attributed to the poor penetration of blue light into the BS cells. Thus, this results in insufficient production of ATP in the cells to match the rates of M cell CO2 pumping (Evans et al., 2007). This suggests that cooperation of both BS and M cells is required for high photosynthetic activity in C4 leaves; however, further experiments are needed focusing on the distribution of the metabolites to fully understand this process.
. Main Conclusions
PSII in maize BS chloroplasts is not equivalent to PSII in the stroma membranes of C3 plants; it exists in the dimeric form and forms LHCII-PSII supercomplexes. Also, it is susceptible to photoinhibition, supporting the hypothesis that photoinhibition is not exclusively dependent on the electron transport rate.
Mesophyll chloroplasts have “state transitions,” which are known to exist in C3 plants, whereas BS chloroplasts exhibit a uniquely permanent State 2, independent of light quality, where LHCII is associated with PSI.
In the stroma lamellae and margin regions of the maize, the M chloroplasts have PSI-LHCI-PSII-LHCII megacomplexes and PSI-LHCI-LHCII supercomplexes, formed under both low and high light growth conditions. The role of these complexes is in excitation-quenching for PSI and turnover for PSII.
Regulation of the distribution of light intensity between M and BS cells shows that co-operation between both metabolic systems determines effective photosynthesis and reduces the harmful effects of high light on the degradation of PSII.


