. Introduction
The Cyperaceae family, commonly referred to as the sedge family, is a globally found herb family that displays an outstanding range of ecological adaptations, spanning from sea level to alpine heights (Batool et al., 2020). Cyperaceae is the seventh largest family in angiosperms and the third most diversified family of monocots. Cyperaceae is an herbaceous plant family with approximately 5,700 species organized into two subfamilies, 24 tribes, and 90 genera. Of its 90 genera, 79 are members of the early-developing Cyperoideae subfamily and 11 of the late-developing Mapanioideae subfamily (http://legacy.tropicos.org/Name/42000356?projected=32; Larridon et al., 2021a). Despite its global distribution, this family has a high degree of diversity in the tropics (de Oliveira Silva et al., 2023). Most species are found in Africa and the Neotropics (Kukkonen, 2001). There are 179 species in 22 genera of the family Cyperaceae in Pakistan, the majority of which are weedy (Kukkonen, 2001).
Cyperaceae species can develop into thick rhizomatous or stoloniferous clusters that are caespitose annual or perennial herbs. They can be found in a range of environments, including dry and waterlogged areas, but they are absent from the Antarctic mainland. Despite their common misconceptions of being uniform and grass-like, sedges are highly variable morphologically (Larridon et al., 2022). It is regarded as a cosmopolitan family and the most prevalent type of wetland vegetation (Bezerra et al., 2023). The habitat of these species varies from grime salinities to hyperregulated water, but most are present in wetlands or poor soil (A. Mumtaz et al., 2021). Typically, they are herbaceous, either annual or perennial, frequently have rhizomes, and are occasionally stoloniferous. Cyperaceae has reduced flowers, and their vegetative anatomy has been shown to be helpful in assessing the presence of similar anatomical traits among various taxa and infrageneric classification of the family (Silva et al., 2019).
Cyperaceae leaves display the typical morphology of monocotyledons, with leaf blades and sheaths that are typically closed in this family (Alves-dos-Santos et al., 2023). The classification of the family Cyperaceae includes, among other things, the spikelet and inflorescence structures. However, it might be difficult to examine because the spikelet is very small, and the inflorescence has a complex structure. Stems are typically trigonous, solitary to densely fascicled, radiately distributed, strong or slender, sturdy or obliquely ascending, and rosette-like in form. Basal and cauline, usually ranking leaves, contain sheaths or blades. Leaf blades are often concave, broad or narrow, linear, grass-like, or involute. Sheaths can be either closed or open. Ligules are frequently observed occasionally on the opposing sides of the leaf blades. There are no petioles, or the blades are essentially limited to a pseudopetiole (Xu et al., 2017), and few taxa, tribes, and subfamilies are uncertain. In addition to conducting the first systematic study of sedges, C. B. Clarke (1893) grouped the 449 species from the Flora of British India into 28 genera (Aryal, 2023).
This family has diverse reproductive and seed dispersal structures, and this morphological difference is used to define taxon limits and to observe the taxonomic complexity of genera such as Cyperus and Carex, and a broad range of dispersal vectors of this family, such as birds and ants (Larridon et al., 2021a). Cyperaceae fruits can fall under the mother plant to produce long-lasting seed banks or they can be adapted for dispersal by water, wind, insects, birds, and mammals (Leck & Schütz, 2005). The Cyperaceae family has intrinsic characteristics, such as high reproductive output, vegetative proliferation, and extended seed dormancy, which enhance the spreading and expansion of the population after any disturbance and evolve as colonizers of disturbed habitats (Bryson & Carter, 2008). Within Cyperaceae, two major clades that correspond to the subfamilies Mapanioideae and Cyperoideae have some differences, whereas Cyperoideae is significantly more diversified in terms of species richness, morphology, and ecology. Mapanioideae are primarily composed of broad-leaved tropical forests beneath herbs (Larridon et al., 2021b).
Cyperaceae has achenes that are quite large and typically prominently sculptured, cylindrical spikes that are sometimes broader than the culms, and spikelet scales with 15 or more prominent longitudinal veins (González-Elizondo & Peterson, 1997). A higher order of spicate, paniculate, or umbellate inflorescences is formed from inconspicuous flowers, which are grouped into spikelets. Flowers can be perfect or imperfect, and plants become monoecious (or very rarely dioecious) when they are imperfect (Cronquist, 1981).
Taxonomists are interested in information regarding the structure of the leaf epidermis. The diversity of natural habitats or genetic variations may cause variations in epidermal features among species (Hameed et al., 2020). Foliar epidermal characteristics have very important analytical properties, such as the size and shape of stomata, guard cell morphology, number of subsidiary cells, and their length and width (Hussain et al., 2019). It is valuable, both theoretically and practically, to observe the microstructure of the leaf epidermis. To study leaf growth and function, as well as the classification of plant species, the micromorphology of leaf epidermis is quite useful (Yuan et al., 2020). LM was used to examine the micromorphology of the foliar epidermal layer using microscopic techniques for taxonomic analysis. Transmission light is frequently utilized as a light source in light microscopy (Abid et al., 2023). Therefore, it is difficult to underestimate the significance of anatomical methods in taxonomic research. Taxonomic monographs without microscopic details of the epidermal anatomy are insufficient (Abbas et al., 2022). The identification of any species depends on the morphology of the plants (Khan et al., 2023; Ullah et al., 2018). Various quantitative and qualitative morphological investigations have been conducted on many families (Attar et al., 2019).
The sedge family (Cyperaceae) is well known to be a taxonomically challenging family that can be observed by considering the anatomical characteristics of vegetative organs for taxonomic purposes (Metcalfe, 1971). Although morphological and molecular approaches have been used to study the interactions among sedges in extensive detail, the linkages among sedges are still incompletely understood (Bouchenak-Khelladi et al., 2014). Previous research has been conducted on the anatomy of several Cyperus species and other Cyperaceae genera (Amini Rad et al., 2008). A literature survey has shown that the foliar anatomy of Cyperaceae has not yet been comprehensively studied. This study aimed to examine the leaf anatomy of selected cyperaceous species using light microscopy to reveal significant taxonomic features. The analysis of leaf structures, epidermal cell morphotypes, and stomatal complex and trichome micromorphology revealed characteristics to construct taxonomic keys based on epidermal anatomy, leading to the identification and delimitation of Cyperaceae species. The species were selected for the study based on their diverse ecological niches and taxonomic relevance within the Cyperaceae family, which enabled a comprehensive examination of foliar anatomical variations.
. Materials and methods
Plant material, identification, and herbarium deposition
Cyperaceae species selected for this study were chosen based on their representativeness of diverse taxonomic groups within the Cyperaceae family and their occurrence in various ecological habitats across the studied regions. Moreover, selection was aimed at capturing a broad spectrum of foliar anatomical traits by including species with distinct morphological and ecological characteristics. The sampling sites were strategically chosen from Dera Ismail Khan, Bannu, Lakki Marwat, and Islamabad to ensure geographic and environmental diversity. Cyperaceae samples were collected from various regions of Dera Ismail Khan, Bannu, Lakki Marwat, and Islamabad from March to August 2023, after extensive fieldwork (Table 1). Plant identification was performed using the online Herbaria database The Flora of Pakistan (http://www.efloras.org), Plants of the World Online (https://powo.science.kew.org), and related portals such as The Plant Net (https://identify.plantnet.org). The dried, pressed, mounted, and labelled plant specimens were placed in the Herbarium of the Plant Sciences Department, Faculty of Biology, Quaid-e-Azam University Islamabad (ISL).
Table 1
Checklist of cyperaceous plant sampling, herbarium accession, and GPS coordinates.
Light microscopy
For epidermal leaf anatomy, leaves from the fresh and preserved collections were used. A modified method was used to prepare leaf samples, as described by Ahmad et al. (2010). Initially, leaves were carefully placed in a test tube containing 30% nitric acid and 70% lactic acid. It was then boiled for 10–15 minutes when the leaves were softer and it was simple to peel off the outer layer. Subsequently, the boiled material was placed in a Petri plate containing dilute water where slides of the adaxial and abaxial epidermis appeared. The isolated epidermis was cleaned with a droplet of lactic acid before mounting the piece on a slide using coverslips. Three to four samples of the adaxial and abaxial surfaces were prepared for each type of plant taxon. The slides were then examined under a light microscope for quantitative analysis (Glime & Wagner, 2013). At 40× magnification, 10–12 readings for each species were obtained using a Meiji (MT 4300H) light microscope. Microanatomical features such as the length and width of epidermal, subsidiary, guard, and stomatal cells were considered (Abbas et al., 2022), and photography of both adaxial and abaxial images was performed using a C-ME1 model camera fitted with a Leica microscope. All quantitative data, such as mean and standard deviation, were measured using SPSS software.
Stomatal index determination
The number of epidermal cells and stomata was counted under the same ocular conditions, and an average of five was recorded. The stomatal index was determined using the formula described by Beerling and Kelly (1997).
Statistical analysis
Different microanatomical features, such as the length and width of epidermal cells, stomata, guard cells, subsidiary cells, and stomatal pores, were analyzed quantitatively. Five consecutive values were used to calculate the mean and standard error for each characteristic (Zaman et al., 2023). Principal Component Analysis (PCA) was employed to obtain the values of the different characteristics. The statistical software IBM SPSS 16.0 Statistics was used for these calculations. Relationships and comparisons between different species were determined using graphs in MS Excel.
. Results
Foliar anatomy
Using a light microscope, the foliar epidermal anatomy of 18 Cyperaceae species from different regions of Pakistan was investigated. Both qualitative and quantitative characteristics, including shape, type, pattern of appearance, stomatal complex, size, and the number of epidermal cells were observed (Figure 1, Figure 2, Figure 3, Figure 4, Table 2, Table 3).
Figure 1
Field collection and foliar anatomical micrographs (author: Arfeen Zahra) of epidermal cells (EC), stomata (St), anticlinal walls AW of Cyperaceae species. (A–C) Cyperus rotundus; (A) field photograph; (B) abaxial surface; (C) adaxial surface; (D–F) Cyperus nutans, var. ,eleusinoides; (D) field photograph; (E) abaxial surface; (F) adaxial surface; (G–I) Cyperus alterniflorus; (G) field photograph; (H) abaxial surface; (I) adaxial surface; (J–L) Cyperus longus; (J) field photograph; (K) abaxial surface; (L) adaxial surface.

Figure 2
Field collection and foliar anatomical micrographs (author: Arfeen Zahra) of epidermal cells (EC), stomata (St), anticlinal walls AW of Cyperaceae species. (A–C) Carex flacca; (A) field photograph; (B) abaxial surface; (C) adaxial surface; (D–F) Fimbristylis bisumbellata (Forssk.) Bubani; (D) field photograph; (E) abaxial surface; (F) adaxial surface; (G–I) Cyperus nutans; (G) field photograph; (H) abaxial surface; (I) adaxial surface; (J–L) Cyperus esculentus; (J) field photograph; (K) abaxial surface; (L) adaxial surface.

Figure 3
Field collection and foliar anatomical micrographs (author: Arfeen Zahra) of epidermal cells (EC), stomata (St), anticlinal walls AW of Cyperaceae species. (A–C) Cyperus esculentus; (A) field photographs; (B) abaxial surface; (C) adaxial surface; (D–F) Cyperus exaltatus; (D) field photographs; (E) abaxial surface; (F) adaxial surface; (G–I) Cyperus malaccensis; (G) field photograph; (H) abaxial surface; (I) adaxial surface; (J–L) Cyperus iria; (J) field photograph; (K) abaxial surface; and (L) adaxial surface.

Figure 4
Field collection and foliar anatomical micrographs (author: Arfeen Zahra) of epidermal cells (EC), stomata (St), anticlinal walls AW of Cyperaceae species. (A–C) Cyperus difformis; (A) field photograph; (B) abaxial surface; (C) adaxial surface; (D–F) Fuirena pubescens; (D) field photograph; (E) abaxial surface; (F) adaxial surface; (G–I) Cyperus niveus; (G) field photograph; (H) abaxial surface; (I) adaxial surface; (J–L) Cyperus flavescens; (J) field photograph; (K) abaxial surface; (L) adaxial surface; (M–O) Cyperus alulatus; (M) field photograph; (N) abaxial surface; (O) adaxial surface.

Table 2
Quantitative foliar epidermal anatomical features of cyperaceous taxa.
Table 3
Quantitative anatomical characteristics of leaf epidermal surfaces of cyperaceous taxa. (Keynotes: + = Present; – = Absent).
Epidermal cells anatomy
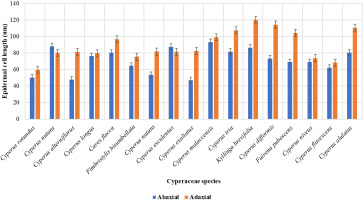
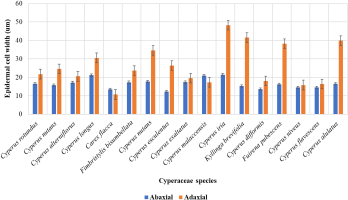
The current study was conducted based on the quantitative analysis of both the width and length of the leaf epidermal cells found on the upper (adaxial) and lower (abaxial) surfaces. The qualitative attributes examined were epidermal cell appearance, AW pattern, and stomatal types on abaxial and adaxial surfaces. However, the measurements showed that most of the epidermal cells on the lower surface had the greatest length, whereas the cells on the upper surface had the maximum width. Cyperus melaccensis has the largest epidermal cells (93.26±0.74 µm) on the abaxial surface while Cyperus iria has the largest epidermal cells (107.32±0.96 µm) on the adaxial surface. Cyperus iria has the highest width of epidermal cells in both the abaxial (21.42±0.95 µm) and adaxial surface (48.26±0.79 µm) as shown in Figure 5, Figure 6 and Table 2. On the abaxial surface, the anticlinal walls of epidermal cells were wavy in Cyperus rotundus, Cyperus nutans, var. ,eleusinoides, Carex flacca Schreb, Cyperus esculentus, Cyperus malaccensis, Kyllinga brevifolius, Fuirena pubescens, and Cyperus alulatus. Sinuous-wavy anticlinal walls were observed in Cyperus difformis and Cyperus niveus. Epidermal cells are present in linear rows with large and few small cells. Epidermal cells are rectangular to polygonal and vary in size from small to large. Only the longer cells were measured (Table 3).
Stomatal complex
The stomatal complexes of the 18 studied species were examined under a light microscope. Variations in the width and length of stomatal pores and guard cells were observed on both the upper and lower leaf surfaces. All species studied were found to have stomata only on the abaxial surface, and they were paracytic, with only two subsidiary cells surrounding them (Table 3). Cyperus iria has the stomata and guard cells with the largest length of (53.47±0.88 µm) and (50.08 ±1.01 µm) respectively. Kyllinga brevifolius has the highest width of stomata and guard cells with (36.56±1.016 µm) and (14.96±1.03 µm) respectively. Cyperus nutans have the smallest length of stomata (28.50±1.51 µm) and guard cells (20.86±1.22 µm). Like all monocots, members of the Cyperaceae family have dumbbell-shaped stomata (Table 2). Trichomes were only observed in Kyllinga brevifolius. This combination of features may serve as a distinguishing characteristic of the species within this family. The largest subsidiary cell (67.71±1.59 µm) was observed in Kyllinga brevifolius while the widest subsidiary cell (27.38±0.98 µm) was found in Cyperus iria. Most species have 1–4 rows of stomata in the bands on the lower surface in almost all species.
Stomatal pores
In the Cyperaceae family, the stomatal pores have a unique shape, which is commonly referred to as “horseshoe-shaped” or “annular.” These pores were curved, resembling a horseshoe, with guard cells forming a ring around the pore. This shape is distinct from species within the Cyperaceae family and differs from the more common circular or elliptical shapes found in other plant families. The largest stomatal pore (21.77±0.99 µm) was observed in Furiena pubescens. Cyperus alternofolius have the widest pores (4.83±1.21 µm) whereas, Cyperus rotundus has the smallest pore (0.66±0.11 µm). In this study, we have identified consistent anatomical traits in the leaf epidermis of certain species (Table 2, Table 3).
Stomatal index
Cyperus nivius showed the highest stomatal index (46.15%) on the abaxial surface, whereas Kyllinga brevifolius showed the lowest (20%) stomatal index on the lower surface.
Dichotomous taxonomic key based on Cyperaceae foliar anatomical traits
Abaxial epidermal cells
Anticlinal wall pattern of abaxial epidermal cells
Number of epidermal cells (abaxial)
Number of stomata (abaxial)
Stomata type
Stomatal pore shape
Number of stomata (abaxial)
Adaxial epidermal cells
Anticlinal wall pattern of adaxial epidermal cells
Number of stomata (abaxial)
Number of stomata (abaxial)
Number of stomata (abaxial)
Shape of epidermal cells (adaxial)
Dendrogram and PCA clustering tool analysis
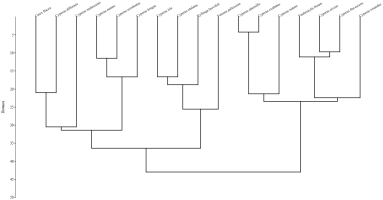
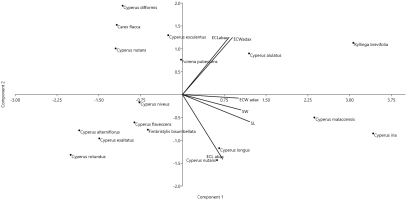
Cluster analysis based on Euclidean distance was performed using the analytical pollen data of selected Cyperaceae species. Two major associations were formed: C1 and C2. Association C1 consisted of ten species. These species have similarities in their colpi length and width (Figure 7). PCA was performed using quantitative microanatomical data from cyperaceous taxa. PCA, principal component analysis; ECL, epidermal cell length; ECW, epidermal cell width; SL, stomatal length; SW, stomatal width. Association C2 consists of seven species and these associations have similarities based on epidermal cell length, epidermal cell width, stomatal length, stomatal width PCA analysis was used on the foliar epidermal quantitative mean data of cyperaceous species to determine the variance among different variables in the context of eigenvariables. PCA illustration showed that the length of epidermal cell on the PC1 axis of C. esculentus and width of epidermal cell of C. alulatus was characterised by a higher values, whereas on PC2 a higher value in terms of epidermal cell length was observed with reference to C. longus (Table 4 and Figure 8).
. Discussion
This study systematically investigated the qualitative and quantitative characteristics of the Cyperaceae family. Qualitative features, such as the shape of epidermal cells, anticlinal wall, types of stomata, and trichomes on both adaxial and abaxial surfaces, were studied. Quantitative features included measurements of the length and width of leaf epidermal cells, stomata, stomatal pores, subsidiary cells, and trichomes, along with the calculation of stomatal indices for both the adaxial and abaxial surfaces. These findings provide valuable insights into the taxonomic identification of plant species in the Cyperaceae family. The foliar epidermal characteristics analyzed in this study are significant for identification and species delimitation within the family Cyperaceae. Furthermore, a study revealed that anatomical features are constant, adaptable, and not subject to change with environmental variations (Ullah et al., 2018).
Under a light microscope, the cells appeared elongated with varying thicknesses and outlines, ranging from irregular to rectangular, and even polygonal. The shape of the anticlinal wall may be straight, slightly wavy, or strongly undulated, similar to the previous work of Ahmad et al. (2010). According to Bercu (2019), sinuous epidermal anticlinal walls were observed in Cyperus alternifolius, whereas straight anticlinal walls were observed in this study. The epidermal cells exhibited variation in size between the adaxial and abaxial surfaces, with the cells on the adaxial surface being significantly larger than those on the abaxial surface and this observation by Hameed et al. (2012) aligns with the findings of the current study.
According to Odedeji and Adedeji (2015), the paracytic type of stomata, where guard cells are surrounded by two subsidiary cells, has been found in all the species studied. All species were hypostomatic, meaning that they had stomata only on the lower surface. Mumtaz et al. (2021) observed stomata on both the upper and lower surfaces of Cyperus rotundus, Cyperus iria, Cyperus longus, Cyperus diformis, Cyperus alternifolius, Cyperus nutans, and Cyperus esculentus; however, in this study, no stomata were observed in these species on the upper surface. Similar results were observed by Oh and Park (1997) for the Fimbristylis species. According to Parvez et al. (2023), in C. nivius the upper epidermal cells are larger than the lower epidermal cells and stomata were only observed on the lower surface, similar to this study. Epidermal and stomatal cells were observed in linear rows oriented parallel to the major veins, which is similar to the previous work of Rudall (2023). Hameed et al. (2012) observed no stomata in Cyperus species on the abaxial surface which is opposite to the current results as stomata were only observed on the abaxial surface just like observed by Zarinkamar (2006). Regarding Carex, the results involving stomata and epidermal cell length and shape were consistent with the previous work of Bugg et al. (2013). In a study conducted by Odedeji and Adedeji (2015) on the Cyperaceae family, trichomes were found only in Kyllinga species and not in any Cyperus species; a similar result was observed in the current study. Trichome micromorphology provides important information regarding species, genera, tribes, and subfamily relationships. The length and width of the stomata, guard cells, epidermal cells, and subsidiary cells of the studied Cyperus species vary greatly from those observed by Hamid and Al-Garaawi (2023).
. Conclusion
This study examined the foliar leaf anatomical traits of Cyperaceae species collected from diverse ecological sites using light microscopy. Both qualitative and quantitative micromorphological epidermal traits of Cyperaceae species play a significant role in describing and defining correct taxonomic identification. Furthermore, the taxonomic keys developed based on the epidermal micromorphology of Cyperaceae species clearly differentiated the species and distinguished each studied species character to use them in the future for further systematic classification and visualization of leaf traits using scanning electron microscopy.
Acknowledgment
This research was funded by the Researchers Supporting Project, number (RSP2024R306), King Saud University, Riyadh, Saudi Arabia.